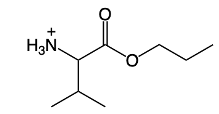
In the reactions of amino acids, we're first going to take a look at esterification. Now, here recall that Fischer esterification is an acid catalyzed esterification of a carboxylic acid. And the carboxylic acid group of amino acids can also undergo Fischer esterification. If we take a look here, we have our amino acid, a generic form of it. If we undergo esterification, we're going to react it with an alcohol.
And in the process, we've created an ester here, so we have OR right here. So we've changed our carboxylic acid group into an ester group. Now if we go the opposite way and we use HCl or Sulfuric acid, we would change it back into the parent carboxylic acid. This would be done under hydrolysis. So we can think of these as acid hydrolysis setup.
Now, we're going to say here it's the same nucleophilic acyl substitution mechanism as we detailed within our carboxylic acid derivatives chapter. So take a look back at that section to see how exactly we worked out the mechanism. But again, remember, we can change our carboxylic acid to an ester through an esterification process. Amino acids having our carboxylic acid groups can also undergo the same type of process.