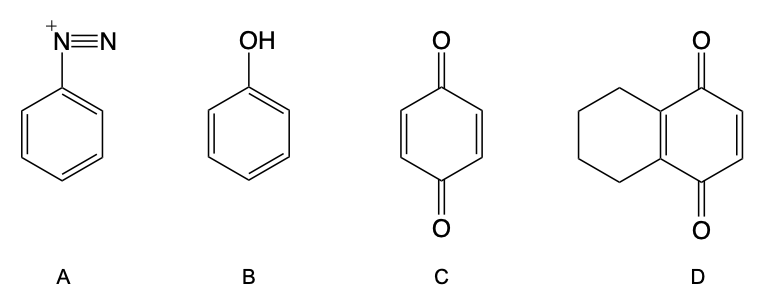
Hey, everyone. So in this video, let's talk about the oxidation of Phenol into Quinone. Now, Phenol can be oxidized into Quinone in the presence of the strong oxidizing dichromate, which is Cr2O7-2. Now, Quinone itself is a conjugated 6-membered ring that possesses carbonyls that are either 1,2 or 1,4 to each other. Now, quinone is also named as cyclohexadienedione.
So cyclo is a ring, hexa is 6 carbons, diene is 2 double bonds, and dione is 2 carbonyls. Now, if we compare the oxidation of a tertiary alcohol to phenol, remember with tertiary alcohols, there is no alpha hydrogen on this carbon. This is the alpha carbon that possesses the OH. There's no hydrogen on it, so no oxidation can take place, so we would write no reaction. Phenol, though, is uniquely different.
We can oxidize it, creating our quinone. Here we're talking about 1,4-quinone, which means our carbonyls will be 1,4 to each other. And what was the other one? And remember, quinone is a diene as well, so we'd have 2 double bonds. This product here is called 1,4-benzoquinone or simply 1,4-quinone.
Just remember that tertiary alcohols do not undergo oxidation, but the unique ability of phenol exists where it can be oxidized. Here, it creates our quinone, which again is a 6-membered ring that's conjugated because it's double, single, double. We have 2 carbonyls and 2 double bonds.