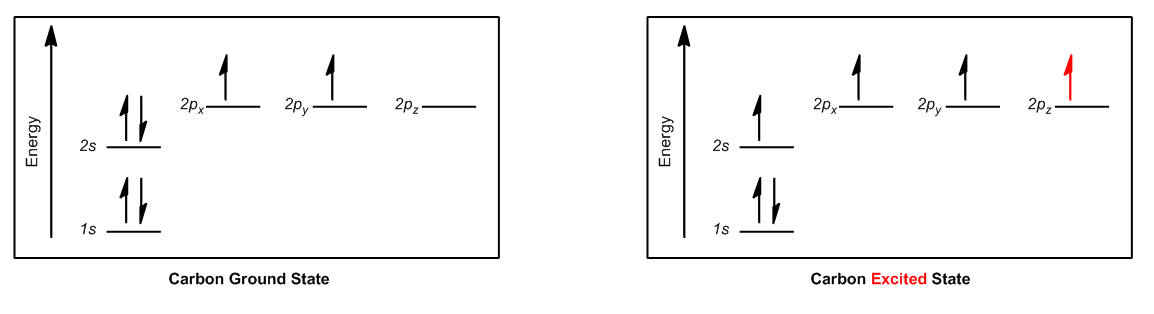
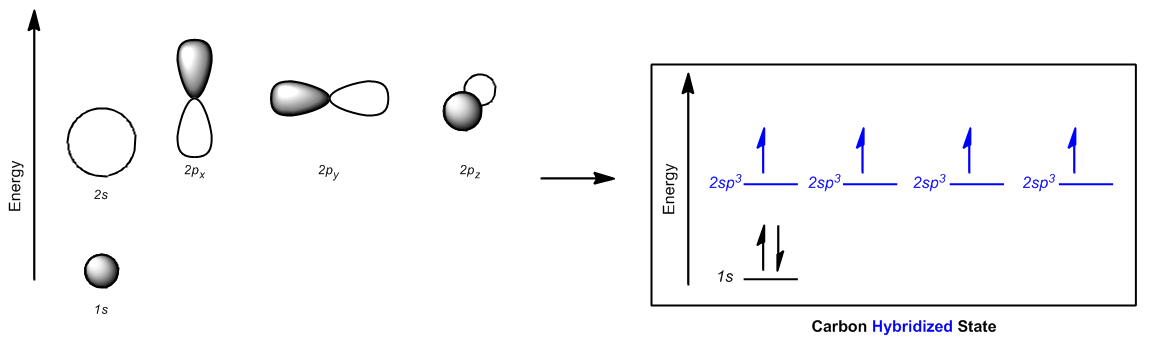
Now that we understand the theory behind hybrid orbitals, let's go ahead and just figure out the rules that are going to help us determine what type of orbitals we're dealing with for different atoms. Hybridization, it turns out, instead of looking at just the electrons like we were doing, it can be determined in a much easier way. It can be predicted by the number of what we call bond sites on an atom. Okay? Bond sites, just so you know, some people refer to these as groups. But I think "groups" is a really general word. It's like it's easy to forget what a group is, but a bond site is really way more specific. So a bond site is going to be equal to any atom or lone pair. So basically, any time that an atom is attached to another atom, regardless of what type of bond it is, it could be a single bond, double bond or triple bond, all of those count as just one bond site. Why? Because there's only one place that it's attached to an atom. Then a lone pair counts as another bond site because that's a place that it could form another bond if it wanted to. So what I want to do is I want to go through a hybridization summary and help you guys realize how just by looking at bond sites, we can determine everything about these atoms in terms of their hybridization.
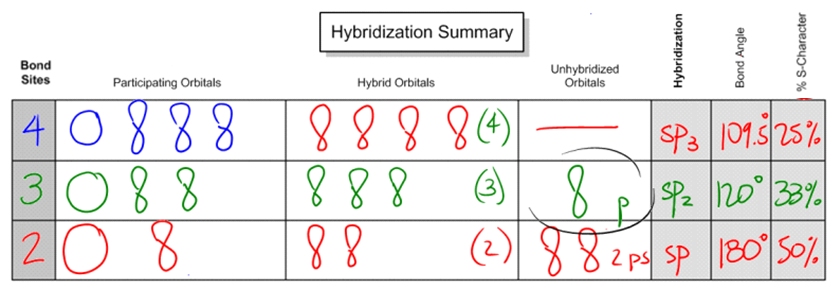
So let's start off with 4 bond sites. What happens if I have a combination of atoms and lone pairs that gives me 4 bond sites? For example, let's say that I just have a carbon with 4 bonds on it. All of these 4 single bonds. All of these bonds represent atoms that it's attached to. Well, if I have a carbon that looks like this, the participating orbitals are going to be that I have the 2s orbital. Remember that I have that 2s orbital that's spherical that is going to hybridize. Then I'm going to blend that with 3 of the p orbitals. Does that make sense so far? Remember that the p orbitals were of higher energy, the s was of lower, but I blend them all together for hybrid orbitals. So what that means is that instead of getting these orbitals of different energies, what I wind up getting is 4 orbitals of the same energy, or those degenerate orbitals. And these orbitals look a little bit different. They're kind of a combination of the p and the s. So what winds up happening is that instead of just looking like a peanut or a sphere, they look kind of like this with one big side and one small side and you get 4 of these. Okay. So basically, oops, that one looks a little bad. I'm going to draw it again. So basically, all of these orbitals combine together and what I wind up getting is 4 of the hybridized orbitals. We're going to talk more about what they are. Unhybridized orbitals actually is none because all of these orbitals participated together and they all blended together to make these 4 degenerate orbitals. Does that make sense so far that they all have the same energy? So now if I was talking about the hybridization, what would we call these orbitals? Would we call them p? Would we call them s? No. We would call them a combination of what they are. So remember what the name would be? It would be sp3. The reason we would call it sp3 is because I have one s and 3 p's combining together to make these orbitals. So what I would get is four sp3 orbitals that are brand new orbitals that I can use. Does that make sense? I hope so.
Well, now let's talk about the bond angle and the s character because these are common things that professors want you to know about the hybridization. Bond angle has to do with how far apart these 4 orbitals can get from each other. And it turns out that if I was on a two-dimensional space, so let's say that this paper I had 4 different orbitals on it and I wanted to get them as far apart from each other as possible, the furthest would be 90 degrees like I have here. Notice that here I drew this at a 90-degree angle. Okay? First would be 90 degrees. But atoms don't exist on a plane. They don't exist on a page. They actually exist in 3D. So in 3D, they can actually move in different directions. So what I could do is I could make 2 of them come out of the plane. If they come out of the plane, what that means is that the bond angles are going to turn into the furthest way they can get from each other is going to be 109.5. This should be an angle that you kind of remember from general chemistry. Okay? The way that you can think of it is that basically 2 of them are going front and back. So imagine that my hands are 2 of the bond sites and then 2 of them are going side to side like my legs. I know you can't see my legs, but I'm just explaining that if my legs were spread apart and if my hands were spread apart this way, that's what a tetrahedral or that's what a 109.5 would look like. I'm going to explain what the shape is in a second. Then finally, what's the s character, percent s character? That doesn't even make sense. Well, all that is, is it's a percentage of s. What part of the entire thing is the s? So I have 4 orbitals total. One of them is s. So overall, what percentage is made out of s? 25%. So let's go ahead and write that in. What I'm trying to say is that if you have 4 and one of them is the s, your percentage of s character is going to be 25%. Why is this important? Because when we get to the acids and bases chapter, we're going to need to know that. We're actually going to need to know the s character to predict some types of acidities.
Now let's move on to the next situation, which is: What if we don't actually have 3 bonds 4 bond sites? How about if we have 3? This is also common. What if I have a molecule that looks like let's say C with a double bond O and 2 carbons on the side. Notice that here, I know that you can't really see the O that well, but notice that this carbon now would have how many bond sites? Only 3. Because remember that I said that any atom counts as a bond site and any lone pair counts as a bond site. Do I have any lone pairs? No. How many atoms do I have attached to that carbon? Only 3. I have a carbon on one side, carbon on the other side and then the oxygen at the top. So what that means is that in this case, I'm only going to get 3 orbitals hybridizing together. The reason is because I only have 3 places where I'm making bonds. Okay? Does that kind of make sense? I only have 3 places where I'm sharing an electron with another atom. So that's the only ones that are going to hybridize. So what I'm going to hybridize is one of the s orbitals, same as before, but now I'm only going to hybridize 2 of the p orbitals. All right. So I have 1 s and 2 p's and what that's going to give me is 3 hybridized orbitals. Does that make sense so far? But now when it comes to unhybridized orbital, is there going to be anything that's not making a bond to anything? Yes, there is. It's going to be one of the p orbitals. One of the p orbitals, I'm just going to put here 1 p or I'm just going to put here p. One of the p orbitals is not going to participate because it's not making a bond to anything, so it's not hybridizing. Okay? So if I were to find a new name for these orbitals, what are they called? What I would call them is just a combination of what they were originally, one of the s's, 2 of the p's, so this would be called sp2. sp2 is the name of these orbitals and I have 3 of them. So basically, in the first example, I had 4 of the sp3s, but in this next example, I only get 3 of the sp2s and then I have a p orbital that's just left over. So I still have 4 orbitals total, but they're not all hybridizing. Okay? Now in terms of bond angles, how far apart can these guys get? They can get 120 degrees apart. Okay? Because of the fact that now I only have 3 orbitals that I have to worry about I only have 3 bonds that I have to worry about moving apart from each other, repelling each other. The furthest away that three things can get from each other is 120 degrees. So what that means is that it's going to be a slightly different bond angle. Then finally, if I were to analyze the s character for this, I would say, What percentage of the total is the s? And the answer is well, the total is sp2. There's only 1 s and there are 3 orbitals total, so it's going to be 33% s character. That shouldn't be so bad. You guys should understand where I'm going with that. It's kind of a pattern.
So now let's do this last one. What if I have a situation where I only have 2 bond sites? So what that means is that, for example, the one that I had with resonance where I had an N on one side and an O on the other. In this case, I have 2 double bonds, but both of them only count as one bond site because it's only attached to one atom. So if I had a situation like this and I'm looking at this carbon right there, how many bonds am I making? 2. So how many orbitals am I going to hybridize? Only 2 because only 2 of these are accepting electrons from other atoms. So I'm going to take an s and I'm going to take a p. Okay? What that means is that when I go ahead and combine these together, these are also going to make those weird looking orbitals, but I'm going to get even less of them. In this case, I'm only going to get 2. Okay? That means in terms of unhybridized orbitals, now I'm going to have 2 p's just left over. Crazy, right? So now I'm going to put here 2 p's. Okay. If I were to go ahead and say what type of hybrid orbitals are these? Well, it would just be a combination of what's coming together, so this would be called sp. And the furthest that two things can get apart from each other because remember now, I only have 2 things repelling, is 180 degrees. And since I only am combining 1 s and 1 p, the s character is going to be 50%. Does that make sense? This is your summary chart. This is what we're going to use to predict hybridizations. All you really have to do is look at the bond sites and that's going to tell you everything else you need to know.