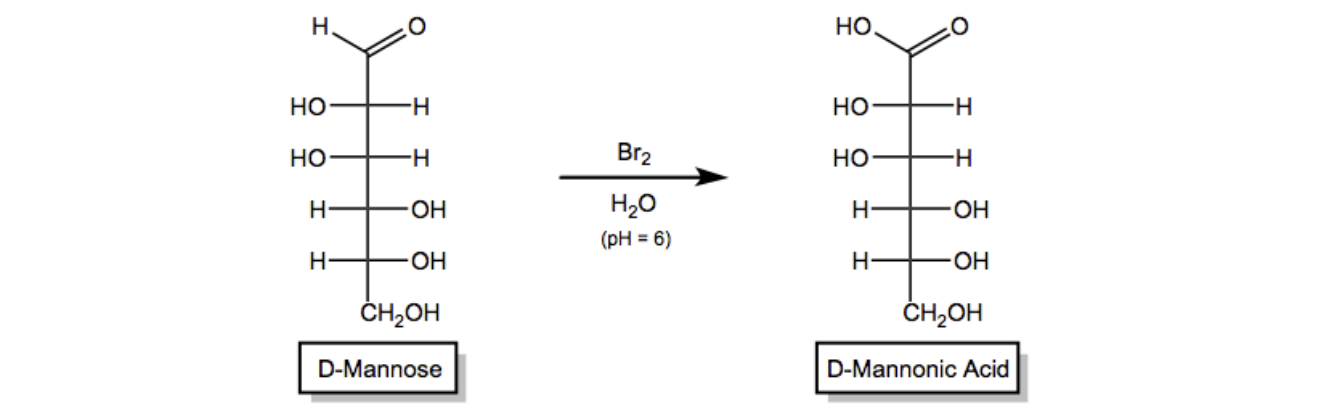
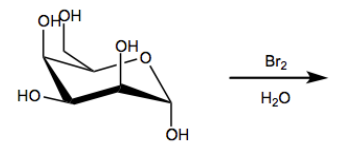
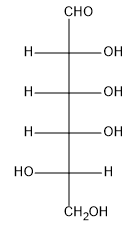
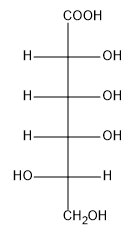
In this video, we're going to learn how to perform a weak oxidation on monosaccharides to yield a functional group called an aldonic acid. Let's get going. So guys, monosaccharides are essentially polyols with carbonyls attached, or with one carbonyl attached. That means they can undergo a series of oxidation and reduction reactions because we have so many reactive functional groups. We have alcohols that can be oxidized, we have ketones that can be reduced, all kinds of stuff. Okay?
Well, it turns out that one of those possible oxidations is called weak oxidation. And what weak oxidation is defined as it's the selective oxidation of monosaccharide aldehydes to carboxylic acid. The selective oxidation of just the aldehyde to carboxylic acid is known as weak oxidation. Now, guys, think about it. All of these different OHs could potentially be oxidized. They could be; this OH could be oxidized to a ketone. This OH is a primary alcohol that could be oxidized to a carboxylic acid. If I use a strong enough oxidizing agent, I could turn this whole thing into ketones, carboxylic acids, etc. But with the special oxidizing agent, I can simply select the very top aldehyde and turn it into a carboxylic acid. Okay?
In fact, this reaction doesn't even work with ketoses because ketoses don't have aldehydes, right? So it's selective for aldehydes to carboxylic acids. And the reagents that we use for this are actually just called bromine water. Okay? So it's bromine in water. This method can be used to differentiate between aldoses and ketoses because it doesn't work on ketoses, you can't do anything with them. It works on aldoses but doesn't work on ketoses. So it's kind of the basis, the principle of this is the basis for some tests of sugars, some sugar tests to test whether you have one type of functional group or another. This is kind of like one of the tests. Okay.
Now, guys, we're in luck. The mecha