Hey, everyone. So in this video, we're going to take a look at Triacylglycerol Molecules. Now, here before that, we're going to say that our glycerol lipids, well, these are just lipids with fatty acid chains attached to a glycerol backbone. If we take a look at our lipids, remember we have hydrolyzable and also non-hydrolyzable portions of this. Here, we're talking about our Glycerolipids. We have waxes and sphingolipids, but we're only paying attention to our Glycerolipids here. Glycerol lipids can be broken down further into our Triacylglycerols and then we have our Phosphoglycerides. When we're talking about our Triacylglycerols, also known as Triglycerides, we can see that they have a glycerol backbone, and we have 3 fatty acids. And they're attached to the glycerol through ester bonds, so ester linkages. Now, fatty acids can all vary. All three fatty acids, they don't have to be the same. They can be, but they can also be different from one another. Now, here if we take a look, we're going to say if our C2 carbon, so this carbon here, it could be chiral depending on the fatty acids at carbon 1 and carbon 3. If we take a look here, we have our chiral center. It's connected to an H. It's connected to this portion here. And then, if we look, this group up here is different from this group down here. Because of that, carbon number 2 is chiral. Now, here if we go further, we can say that Triacylglycerols, they can function as a source of energy and storage, and they store in the form of fatty tissue otherwise known as adipose tissue. And this is happening within animals. So, just remember, we're talking about lipids, we're still looking at our hydrolyzable lipids. We're paying closer attention to our glycerol lipids which break down into our Triacylglycerols as well as our Phosphoglycerides. For Phosphoglycerides, it's almost the same except instead of having 3 fatty acid chains, we only have 2 with the third one being replaced with a phosphate group and an amino alcohol. Alright. So just keep this in mind when we're talking about our Triacylglycerol Molecules.
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect6h 50m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table21m
- 1H NMR:Spin-Splitting (N + 1) Rule17m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams8m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 31m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
Triacylglycerols - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AITriacylglycerols, or triglycerides, consist of a glycerol backbone bonded to three fatty acids via ester linkages. They serve as energy storage in adipose tissue. Fats, typically solid at room temperature, contain saturated fatty acids with higher melting points due to stronger intermolecular forces. In contrast, oils, usually liquid, have unsaturated fatty acids with lower melting points due to weaker interactions. Understanding the structure and properties of these lipids is crucial for grasping their biological roles and implications in nutrition.
Triacylglycerols Concept 1
Video transcript
Triacylglycerols Example 1
Video transcript
It says to draw a triglyceride structure composed of palmitoleic acid for the first carbon, myristic acid for carbon 2, and oleic acid for carbon 3. Now, based on our memory tools, we know that myristic acid is a saturated fatty acid. It has 14 carbons and no pi bonds. Palmitoleic acid has 16 carbons and 1 pi bond, and oleic acid has 18 carbons and 1 pi bond. Additionally, their 1 pi bonds start on carbon 9. This will become important when drawing these structures.
Now, here we're going to say, step 1 is to draw the glycerol molecule and the 3 fatty acids. We're going to place OH groups next to the carboxyl groups of the fatty acid. So let's do that part first. Here we're gonna draw our glycerol molecule. We're gonna say we have CH2, CH2, CH2, and then we have our OH groups. Now we have to draw our fatty acids. Remember, our first one is an unsaturated fatty acid. We're gonna say it has 16 carbons, so 2, 4, 6, 8. Remember the first pi bond happens on carbon 9. So, 9, 10, 11, 12, 13, 14, 15, 16. Next, we have our myristic acid. It's saturated so it has 14 carbons and no pi bonds. So 2, 4, 6, 8, 10, 12, 14. And then finally, oleic acid has 18 carbons. 2, 4, 6, 8, 9 is where we have our double bond, 10, 11, 12, 13, 14, 15, 16, 17, 18. Now instead of OH on glycerol, we're just gonna write an O. So, we're gonna take away these H's here. And do not draw OH on the fatty acids.
So now it's up to us to connect for step 2. We're gonna form ester bonds between the glycerol OH groups and the 3 fatty acids. We're gonna connect the oxygens of the glycerol to the carbonyl carbons of the fatty acids. Doing this will give us our triglyceride. So, we're gonna have our CH2, and then CH2, CH2. Then we have O, O, and then O. Here's our ester linkage. So then we're gonna draw. Remember, we need 16 carbons. So 2, 4, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16. Here, we need 14 carbons. 2, 4, 6, 8, 10, 12, 14. And then here we need 18 carbons. 2, 4, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 carbons. So, this will represent our triacylglycerol molecule or our triglyceride structure. This would be our final answer.
Triacylglycerols Concept 2
Video transcript
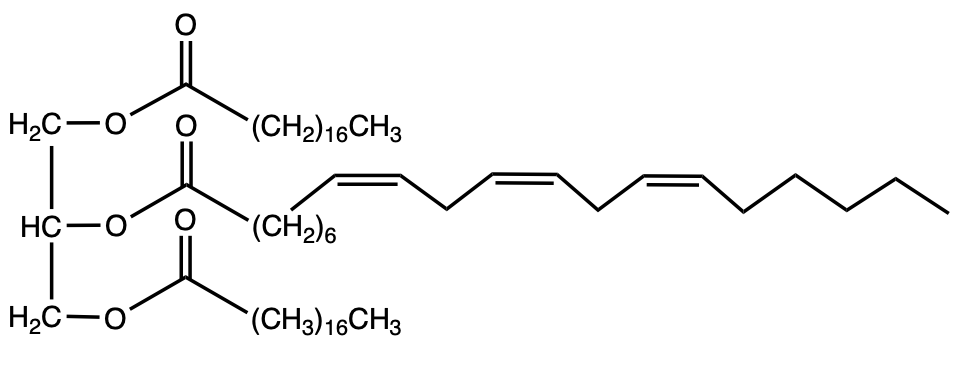
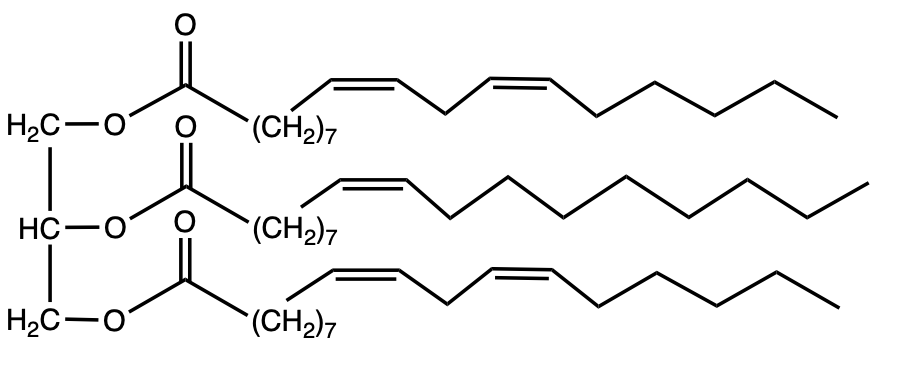
So in our continued discussion of Triacylglycerol Molecules, we now take a look at fats and oils. Now fats and oils can be seen as mixtures of different Triacylglycerols. Here, we're going to say that saturated fatty acids pack more tightly in a solid phase, which is going to help increase interactions between one another. These greater interactions are going to lead to an increase in the intermolecular forces between them, resulting in stronger intermolecular forces. And we're going to say because of this greater increase in intermolecular forces between these nearby molecules, this would translate into higher melting points. Now, if we take a look here at fats and oils, we have fats in terms of animal fats and then we have oils in terms of vegetables. With this, we can talk about melting point, saturation, as well as examples. Now with fats from animals, we're going to say here that the melting point tends to be higher and we're going to say they tend to be solids at room temperature. In terms of saturation, we're going to say that the number of double bonds tends to be low, and then we're going to say it's low in unsaturated fatty acids. A good example here, we have our glycerol backbone, and we have our 3 fatty acids. And we can see only 1 of the 3 has a pi bond involved, indicating very low levels of unsaturation. With oils, and here we're talking about in terms of vegetables, we're going to say they tend to have a low melting point and they tend to be liquids at room temperature. Here, we'd expect to have a higher number of pi bonds and a higher amount of unsaturation from fatty acids. If we take a look here at this example, we still have 3 fatty acid chains, but we can see with these fatty acid chains there's more presence of pi bonds, indicating greater levels of unsaturation. Again, this would mean that these fatty acids would not be able to pack as tightly around one another, so there's greater distance between each other which results in lower interactions, lower intermolecular forces because of lack of interactions, and therefore, a lower overall melting point. Right? So just keep that in mind when we're talking about fats and oils, and we're talking about levels of interactions and how that affects our overall melting point.
Triacylglycerols Example 2
Video transcript
This example question asks, "Which Triacylglycerol would you expect to be liquid at room temperature?" Remember, we have fats, which are indicative of animals, and oils, which are indicative of vegetables and plants. Remember, the more double bonds or π bonds that we possess, the lower our melting point will be, and therefore it is more likely to exist as a liquid at room temperature. If we take a look at option a, we see that we have 1, 2, 3 double bonds or π bonds involved. And if we look at option b, we only have 1 π bond involved. The one most likely to be a liquid at room temperature would have to be option a. It possesses more double bonds, which will result in more kinking of the long fatty acid chain, which will result in less stacking of these structures on top of each other. Meaning they will exist more as a liquid at room temperature and have a lower melting point as a result. All right. Option b is less likely to be a liquid when compared to option a because it has much fewer π bonds or double bonds involved. So, again, in this particular question, option a is more likely to be a liquid than option b because of the presence of these π bonds or double bonds.
Draw a skeletal structure of a triglyceride with linolenic acid (C1) and 2 palmitoleic acids. State whether it would have high or low melting point.
Problem Transcript
Which triacylglycerol is optically active?
Do you want more practice?
More setsHere’s what students ask on this topic:
What are triacylglycerols and how are they structured?
Triacylglycerols, also known as triglycerides, are a type of lipid molecule composed of a glycerol backbone bonded to three fatty acids via ester linkages. The glycerol molecule has three hydroxyl (OH) groups, each of which forms an ester bond with the carboxyl group (COOH) of a fatty acid. The fatty acids can vary in length and degree of saturation, meaning they can be either saturated (no double bonds) or unsaturated (one or more double bonds). This structure allows triacylglycerols to serve as a major form of energy storage in adipose tissue.
 Created using AI
Created using AIHow do saturated and unsaturated fatty acids affect the properties of fats and oils?
Saturated fatty acids have no double bonds, allowing them to pack tightly together, resulting in stronger intermolecular forces and higher melting points. This makes fats, which are rich in saturated fatty acids, solid at room temperature. In contrast, unsaturated fatty acids contain one or more double bonds, introducing kinks that prevent tight packing. This leads to weaker intermolecular forces and lower melting points, making oils, which are rich in unsaturated fatty acids, liquid at room temperature. The degree of saturation directly influences the physical state and melting point of fats and oils.
 Created using AI
Created using AIWhat is the role of triacylglycerols in energy storage?
Triacylglycerols play a crucial role in energy storage within the body. They are stored in adipose tissue, where they can be broken down into glycerol and free fatty acids when energy is needed. The fatty acids are then oxidized in the mitochondria to produce ATP, the energy currency of the cell. This process provides a dense form of energy storage, as triacylglycerols yield more than twice the energy per gram compared to carbohydrates or proteins. This makes them an efficient way for the body to store and mobilize energy.
 Created using AI
Created using AIWhy do fats have higher melting points compared to oils?
Fats have higher melting points compared to oils primarily due to their higher content of saturated fatty acids. Saturated fatty acids lack double bonds, allowing them to pack closely together, which increases the strength of intermolecular forces such as van der Waals interactions. This tight packing results in a solid state at room temperature and a higher melting point. In contrast, oils contain more unsaturated fatty acids with double bonds that introduce kinks, preventing tight packing and resulting in weaker intermolecular forces and lower melting points, making them liquid at room temperature.
 Created using AI
Created using AIWhat is the difference between triacylglycerols and phosphoglycerides?
Triacylglycerols and phosphoglycerides are both types of glycerolipids, but they differ in structure and function. Triacylglycerols consist of a glycerol backbone bonded to three fatty acids via ester linkages, serving primarily as energy storage molecules in adipose tissue. Phosphoglycerides, on the other hand, have a glycerol backbone bonded to two fatty acids and a phosphate group attached to an amino alcohol. This structure makes phosphoglycerides key components of cell membranes, where they contribute to membrane fluidity and function. The presence of the phosphate group and amino alcohol in phosphoglycerides distinguishes them from triacylglycerols.
 Created using AI
Created using AI