Hey everyone, so we're going to start off this course by first talking about the relevance of organic chemistry. At this point, you've taken the course as necessary in general chemistry where you learned about different concepts such as the elements of the periodic table, stoichiometry, titrations, even electrochemistry. The good thing is a majority of this information is not really going to transfer over to organic chemistry. There are only going to be a few concepts mainly around periodic trends that are going to carry over from general chemistry. Right now, don't worry about them too much. We'll cover them when we get to those ideas. Now, back to the relevance of organic chemistry. Well, organic chemistry itself is the chemistry of life. It consists of the study of molecules that are typically created and used by biological systems. So, organic chemistry is important because it involves our everyday lives. If we take a look at this first image, we have the ingredients of a hairspray bottle and I've zeroed in on 4 organic molecules that are found within the ingredients. Right now these molecules don't seem like anything you might recognize. You don't know the number of elements housed within each molecule, but you'll learn about these kinds of ideas and how to interpret a molecule like this later on. But just realize right now that something as simple as a hair bottle is heavily involved in organic chemistry. Now, there are some destructive applications to organic chemistry. Here we have the creation of nerve gases. Here I've circled acetylcholine, acetic acid, and choline itself. These organic molecules are interacting with a biological system here. This is a way of showing how organic chemistry is the chemistry of life, and we're talking about how chemistry interacts with biological systems that also branches off into what we call biochemistry. We're not going to delve too deeply into biochemistry, but just realize that biochemistry is kind of like an extension of these organic chemistry ideas that we're going to discover and talk about this semester. Alright. So for right now just realize that organic chemistry, albeit new to you, is highly relevant in this course and in everyday life.
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect7h 3m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table24m
- 1H NMR:Spin-Splitting (N + 1) Rule22m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams12m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 34m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
Intro to Organic Chemistry - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIOrganic chemistry is the study of carbon-containing compounds, essential for understanding biological systems. It focuses on molecules primarily composed of carbon and hydrogen, known as hydrocarbons. The relevance of organic chemistry extends to everyday products, such as hairspray, and even harmful substances like nerve gases. Key concepts include functional groups, isomerism, and the role of organic molecules in biochemistry. Understanding these principles is crucial for exploring the chemistry of life and its applications in various fields.
Why do we take an entire year learning about Organic chemistry? What makes Organic chemistry so special? Let's find out.
Examples of Organic Molecules
Organic molecules in your everyday life.
Video transcript
Household products have tons of organic molecules in them! It feels awesome when you know what they mean.

Defining Organic Molecules
What is an organic molecule?
Video transcript
So let's just go into recognizing what an organic molecule is. Technically, there are actually a lot of definitions of what an organic molecule is based on the year you're talking about. Back then, they used to have a much different definition. Some books will even say some things that are different. But the majority of the consensus is that an organic molecule, any molecule that contains both carbon and hydrogen, obviously. We know that the chemistry of life is basically built on carbon, at least on Earth, and carbon and hydrogen. The reason I said the whole Earth thing is because some people are like, "Oh, aliens are made out of, like, silicon or something," but, like, whatever. The biology of life has to do with carbons. And then an organic molecule that contains a mixture of hydrogen and carbon only, it doesn't have any other atoms, would be called, you guys know that one? You should know that one. A hydrocarbon.
- Organic Molecule: Any molecule that contains carbon bonded to hydrogen.
- Hydrocarbon: Any molecule that contains only carbon and hydrogen, and nothing else.
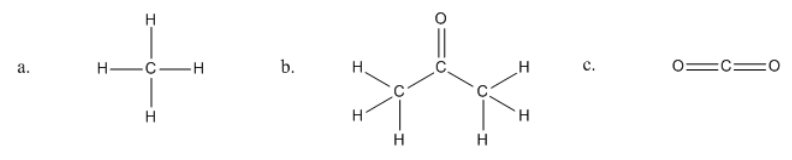
Identifying organic molecules
Video transcript
So hopefully what you said was that letter 'A' is definitely organic. So I'm just going to put an 'O' with a checkbox, and it is because it's made out of carbon and hydrogen. Perfect. Is it also a hydrocarbon? So, I'm going to put the smallest form of carbon called methane. So you might know methane smells bad. It's released like in gas, so sorry. Too much information. But whatever. That is an organic molecule.
How about this next one here? Hopefully, you said that yes, this is organic. Because once again, it has carbon and it has hydrogen. Is it a hydrocarbon? No. This would not be a hydrocarbon. The reason is that it has an oxygen there. See how I have an atom that is not a carbon or a hydrogen? So that means that this is considered just an organic molecule. In fact, this is acetone. So if you, maybe you women use acetone to take your nail polish off, whatever, I hate that smell. It's disgusting. That's what the molecule looks like. We're going to be learning about a lot of really cool molecules. At least, I think they're cool. I have to think they're cool. I'm a tutor. But they are really cool. Hopefully, you'll get to know them too. Don't memorize these names yet. I'm just giving you some information for later.
Then finally, this last one, hopefully, what you said is that this was not organic and also it was not a hydrocarbon. The reason is that I do have a carbon here, but I do not have a hydrogen. There are no hydrogens, so this is not an organic molecule. In fact, this is what we consider can be a little confusing, inorganic carbon, like not organic. This would be a form of inorganic carbon because it doesn't have any hydrogens at all. In fact, this is CO2 or carbon dioxide. So carbon dioxide, you know that's like a greenhouse gas. It goes into the air. That's considered inorganic. We would learn about this a little bit more in gen chem because it has to do more with inorganic and then for organic, we would deal more with the molecules that have hydrogens on them. That's a general rule.
Orgo isn’t so bad, right? Let’s move on to the next topic.
Do you want more practice?
More setsHere’s what students ask on this topic:
What is organic chemistry and why is it important?
Organic chemistry is the study of carbon-containing compounds, which are essential for understanding biological systems. It focuses on molecules primarily composed of carbon and hydrogen, known as hydrocarbons. The importance of organic chemistry extends to everyday products, such as hairspray, and even harmful substances like nerve gases. Key concepts include functional groups, isomerism, and the role of organic molecules in biochemistry. Understanding these principles is crucial for exploring the chemistry of life and its applications in various fields, including medicine, environmental science, and industrial processes.
 Created using AI
Created using AIWhat are hydrocarbons and why are they significant in organic chemistry?
Hydrocarbons are organic molecules that consist solely of carbon (C) and hydrogen (H) atoms. They are significant in organic chemistry because they form the basic framework for more complex organic compounds. Hydrocarbons can be classified into different types, such as alkanes, alkenes, and alkynes, based on the types of bonds between carbon atoms. Understanding hydrocarbons is fundamental for studying more complex molecules and reactions in organic chemistry, as they serve as the building blocks for many biological and synthetic compounds.
 Created using AI
Created using AIWhat are functional groups in organic chemistry?
Functional groups are specific groups of atoms within molecules that have characteristic properties and chemical reactivity. They are the reactive parts of molecules and determine the types of chemical reactions that the molecule can undergo. Common functional groups include hydroxyl (-OH), carbonyl (C=O), carboxyl (-COOH), amino (-NH2), and alkyl groups. Recognizing and understanding functional groups is crucial for predicting the behavior of organic molecules in chemical reactions and for synthesizing new compounds.
 Created using AI
Created using AIHow does isomerism play a role in organic chemistry?
Isomerism is the phenomenon where two or more compounds have the same molecular formula but different structures or arrangements of atoms. In organic chemistry, isomerism is important because it explains the diversity of organic compounds. There are different types of isomerism, including structural isomerism (different connectivity of atoms) and stereoisomerism (different spatial arrangement of atoms). Understanding isomerism helps in identifying and distinguishing between different compounds, predicting their properties, and understanding their reactivity.
 Created using AI
Created using AIWhat is the relationship between organic chemistry and biochemistry?
Organic chemistry and biochemistry are closely related fields. Organic chemistry focuses on the study of carbon-containing compounds, while biochemistry deals with the chemical processes within and related to living organisms. Many of the molecules studied in biochemistry, such as proteins, nucleic acids, carbohydrates, and lipids, are organic molecules. Understanding the principles of organic chemistry is essential for exploring biochemical pathways, enzyme mechanisms, and the molecular basis of life. This relationship is crucial for advancements in fields like medicine, pharmacology, and biotechnology.
 Created using AI
Created using AIYour Organic Chemistry tutors
- There is a small portion of the periodic table that you must know to do organic chemistry. Construct this part...
- a. Draw resonance contributors for the following species. Do not include structures that are so unstable that ...
- a. Draw resonance contributors for the following species. Do not include structures that are so unstable that ...
- a. Draw resonance contributors for the following species. Do not include structures that are so unstable that ...
- Compound X, isolated from lanolin (sheep’s wool fat), has the pungent aroma of dirty sweatsocks. A careful ana...
- In 1934, Edward A. Doisy of Washington University extracted 3000 lb of hog ovaries to isolate a few milligrams...
- Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propo...
- Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propo...
- Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propo...
- Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propo...

