When it comes to fatty acids, we're going to say that most fatty acids contain an even number of carbons, typically 12 to 26, with a generic or general formula of CH3CH2nCOOH. n can vary based on the length of the fatty acid chain. Now, we're going to also say that fatty acids are amphipathic molecules, meaning they have both nonpolar and polar parts.
We're going to say that this depiction, this image on the right represents a fatty acid. The fatty acid itself has a hydrophobic tail, and that's because the tail is made up of only hydrocarbons, only carbons and hydrogens making it nonpolar. And because it's nonpolar, it's hydrophobic. We're going to say the carboxylic acid has a hydrophilic head. Hydrophilic means water-loving. And why is that? Because the carboxylic acid head is polar.
If we take a look here at this image, based on the length of the fatty acid chain, we're going to say this represents Lauric acid. Remember that the hydrocarbon tail would be hydrophobic, and then the carboxylic acid head would be hydrophilic. We say that typically our fatty acids are between 12 to 26 carbons. Only one of these carbons is part of a polar part. The rest of the tail is nonpolar.
So we're going to say here, the larger the hydrocarbon tail then the more nonpolar the fatty acid. Therefore, overall fatty acids are nonpolar. So just remember when looking at a fatty acid we have two parts to it, the hydrophobic hydrocarbon tail and the hydrophilic carboxylic acid head.

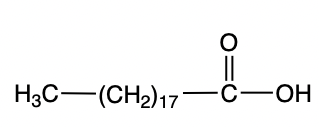 Storic acid
Storic acid Palmitic acid
Palmitic acid Stearic acid
Stearic acid Palmitoleic acid
Palmitoleic acid




