Hey, everyone. In this video, let's take a look at the Woodward-Pfizer rules. Now they themselves represent a set of rules that help to give an estimate for lambda max of conjugated systems. So here we're going to break them down into 4 categories. We have our conjugated diene itself.
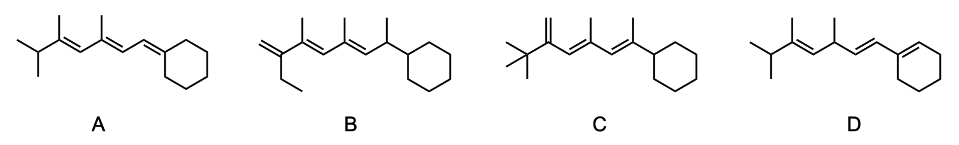
We have our alkyl oxochromic group. We have our exocyclic double bond and then our homo angular diene. So coming back up here to our conjugated diene, we're going to say it has a base value of 217 nanometers. And we're going to say each additional double bond, each additional conjugated double bond will increase the lambda max wavelength by 30 nanometers. So if we take a look at this example here, we have our base, we'll say these 2, that's 217, but then we have 2 additional double bonds.
Two additional double bonds, each one increasing my wavelength by 30 nanometers. So together, that's an additional 60 nanometers. So this will come out to be 277 nanometers. Next, we have our alkyl oxochromic groups. Remember, an oxochromic group is just the nonconjugated portion that's connected to our conjugated portion of the molecule.
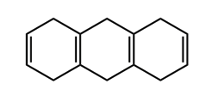
Not all oxochromic groups are alkyl in nature. For example, OH can be an oxochromic group. But here, we're going to say that the alkyl versions of these have a set value. So here we're going to say that in this case, our oxochromic groups are alkyl carbons attached to a conjugated vanillic carbon. We're going to say each one of these increases my lambda max by 5 nanometers.
So if we take a look here at this, we're going to have our base value of 217 nanometers from our 2 double-bonded double bonds that are conjugated. And they're going to say we have 1, 2, 3, 4 alkyl groups directly connected to that conjugated portion. So there are 4 of them, each one contributing an increase of 5 nanometers. So that's 20 nanometers being added, giving us 237 nanometers at the end. Again, this is just an estimate of what our lambda max would be in terms of this particular molecule.
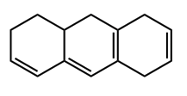
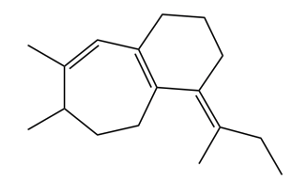
Now next, we go to the exocyclic double bond. So in an exocyclic double bond, we're going to say a double bond with 1 vanillic carbon as part of the ring, so here, and one vanillic carbon extending outside the ring, here. We're going to say each exocyclic double bond also increases my lambda max by 5 nanometers. So if we took a look here, we'd say we have our base of 217 because it's these 2 double bonds. And then we're going to say we have 2 exochromic groups.
So that's 2 times the plus 5 nanometers that they contribute. And then we have this exocyclic double bond, and that includes another 5 nanometers. Alright. So that's going to give me here 232 nanometers. And then finally here, we take a look at our homoanular diene.
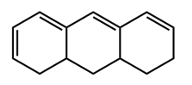
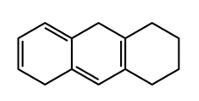
This is just a conjugated diene in the same ring in an s cis conformation. Each one contributes an additional 39 nanometers. So if we take a look here, we're going to say that our homoanular diene is this portion right here. We have a conjugated diene in the same ring and they're both cis. They're pointing in the same direction.
Alright. So here, we're going to say we have our base, which is 217 nanometers. Then we're gonna say next, what do we have? We have an additional double bond here, so that's gonna add an additional 30 nanometers to my wavelength. Then what else are we going to say?
We're going to say here, in addition to that, we have 1, 2, 3, 4, 5, 5 oxochromic groups. So that's 5 times each one contributes an additional 5 nanometers. So that's 25 nanometers. And then we're going to say here that in addition to this, we're going to have our homoanular.
So that's 39 nanometers. And in addition to this, we're going to have here an exocyclic double bond. It's part of a ring, part of it's part of a ring, and it extends outside the ring. That'd be another 5 nanometers. Alright.
So adding all this up together will give us our estimated wavelength, lambda max. So we're going to say 217 plus the 30 gives us 247, plus another 25 here will give us 272, and then another 39 here would give us 311, plus another 5 would give us 316 nanometers roughly. Okay. So that's what we'd say in terms of the lambda max here. This one's a little bit tricky because it's so many moving parts to it.
We have to take into account the fact that we have our base, conjugated diene, the additional double bond, the oxochromic groups, the exocyclic double bond, as well as the homoanular diene. Taking all that into account gives us this rough estimate of the lambda max for this particular compound within the 4th box.