Here are the essential concepts you must grasp in order to answer the question correctly.
E2 Mechanism
The E2 mechanism is a type of elimination reaction where a base removes a proton from a β-carbon while a leaving group (such as a halide) departs from the α-carbon simultaneously. This concerted process results in the formation of a double bond. Understanding the stereochemistry and the requirement for anti-periplanar geometry is crucial for predicting the outcome of E2 reactions.
Recommended video:
Drawing the E2 Mechanism.
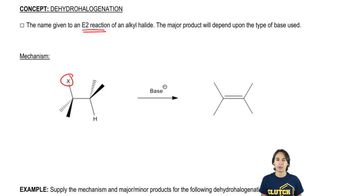
Dehydrohalogenation
Dehydrohalogenation refers to the elimination of a hydrogen halide (HX) from an alkyl halide, leading to the formation of an alkene. This reaction typically requires a strong base and is a key step in synthesizing alkenes from alkyl halides. The choice of base and the structure of the halide influence the reaction pathway and the stability of the resulting alkene.
Recommended video:
The dehydrohalogenation mechanism.
Alkene Stability and Substitution
The stability of alkenes is influenced by their degree of substitution; more substituted alkenes are generally more stable due to hyperconjugation and the inductive effect. This concept is important when predicting which halides will undergo E2 reactions to yield specific alkenes, as the formation of more stable alkenes is favored. Understanding Zaitsev's rule helps in determining the major product in elimination reactions.
Recommended video:
Understanding trends of alkene stability.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 0:38m
0:38m