Here are the essential concepts you must grasp in order to answer the question correctly.
Aromatic Nitration
Aromatic nitration is a chemical reaction that introduces a nitro group (-NO2) into an aromatic compound, typically using a mixture of concentrated nitric acid and sulfuric acid. This electrophilic substitution reaction is crucial for preparing aromatic amines, as it allows for the functionalization of the aromatic ring, which can then be further modified through reduction.
Recommended video:
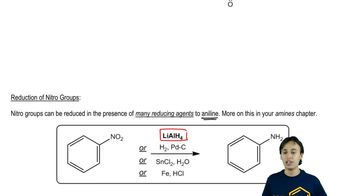
Reduction of Nitro Compounds
The reduction of nitro compounds involves converting the nitro group (-NO2) into an amine group (-NH2). This can be achieved using various reducing agents, such as iron and hydrochloric acid or catalytic hydrogenation. This step is essential for transforming the nitrated aromatic compound into the desired aromatic amine, such as m-aminobenzoic acid.
Recommended video:
Reduction of Nitro Groups
Ortho/Para vs. Meta Directing Effects
In electrophilic aromatic substitution reactions, substituents on the aromatic ring can influence the position of new substituents. Electron-donating groups, like amines, direct incoming electrophiles to the ortho and para positions, while electron-withdrawing groups, like nitro groups, direct them to the meta position. Understanding these directing effects is vital for predicting the product distribution in the synthesis of m-aminobenzoic acid.
Recommended video:
Ortho, Para major products
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 9:12m
9:12m