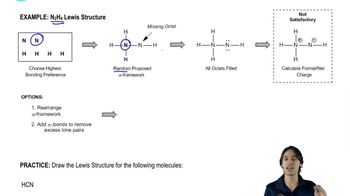
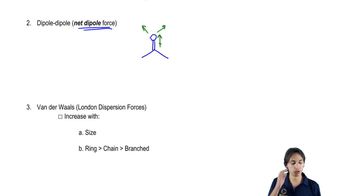
1. From what you remember of electronegativities, show the direction of the dipole moments of the following bonds.
2. In each case, predict whether the dipole moment is relatively large (electronegativity difference >0.5) or small.
a. C—Cl
b. C—H
c. C—Li
d. C—N
e. C—O