Here are the essential concepts you must grasp in order to answer the question correctly.
Ether Cleavage
Ethers, like ethyl ether, undergo cleavage when treated with strong acids such as hydroiodic acid (HI). This reaction involves the protonation of the ether oxygen, making it a better leaving group. The cleavage results in the formation of alkyl halides and alcohols, but the conditions can favor the formation of one product over another.
Recommended video:
Cleavage of Phenyl Ethers Concept 1
Reactivity of Alkyl Halides
In the presence of excess HI, ethyl iodide is favored due to the reactivity of alkyl halides. Ethyl iodide can further react with HI to regenerate iodide ions and promote the cleavage of the ether. This reaction pathway leads to the exclusive formation of ethyl iodide rather than ethyl alcohol, which would require different conditions to form.
Recommended video:
How to name alkyl halides
Equilibrium and Reaction Conditions
The reaction conditions, including the excess of HI and prolonged heating, shift the equilibrium towards the formation of ethyl iodide. The high concentration of HI drives the reaction to completion, minimizing the formation of ethyl alcohol. In contrast, if the conditions were less extreme, both products could potentially form.
Recommended video:
Determining Acid/Base Equilibrium
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: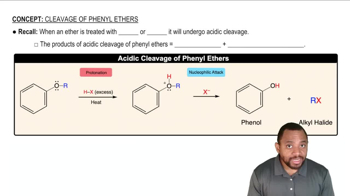

 4:22m
4:22m