Here are the essential concepts you must grasp in order to answer the question correctly.
Stereoisomers
Stereoisomers are compounds that have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of their atoms. This category includes enantiomers and diastereomers, which are crucial for understanding the behavior of molecules in biological systems and their interactions. The presence of asymmetric centers, or chiral centers, in a molecule leads to the formation of these stereoisomers.
Recommended video:
Determining when molecules are stereoisomers.
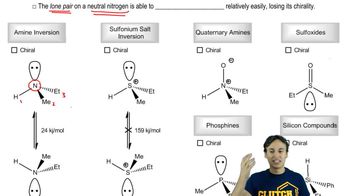
Chirality and Asymmetric Centers
Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image, often due to the presence of one or more asymmetric (chiral) centers. Each chiral center can exist in two configurations, typically designated as 'R' (rectus) or 'S' (sinister). The arrangement of these configurations determines the specific type of stereoisomerism exhibited by the molecule.
Recommended video:
Understanding Other Chiral Atoms
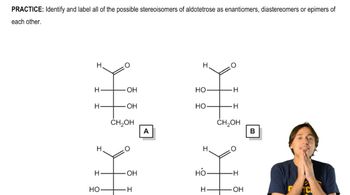
Types of Stereoisomers
The two main types of stereoisomers are enantiomers and diastereomers. Enantiomers are stereoisomers that are mirror images of each other, while diastereomers are not. The question specifically addresses configurations of stereoisomers with two asymmetric centers, leading to classifications based on whether the centers are the same or opposite in configuration, which is essential for understanding their chemical properties and reactions.
Recommended video:
Identifying Types of Stereoisomers
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:41m
1:41m