Here are the essential concepts you must grasp in order to answer the question correctly.
Optical Activity
Optical activity refers to the ability of a compound to rotate the plane of polarized light. This property is characteristic of chiral molecules, which have non-superimposable mirror images. The degree and direction of rotation depend on the specific arrangement of atoms in the molecule, making it crucial for distinguishing between different stereoisomers.
Recommended video:
Mutorotation and Optical Activity
Chirality and Stereoisomers
Chirality is a property of a molecule that has at least one chiral center, typically a carbon atom bonded to four different substituents. Stereoisomers are compounds that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. The presence of chirality in a molecule leads to the existence of enantiomers, which are mirror images of each other and can exhibit different optical activities.
Recommended video:
Reduction Reactions
Reduction reactions involve the gain of electrons or hydrogen by a molecule, often resulting in the conversion of carbonyl groups (like aldehydes or ketones) to alcohols. In the case of D-glucose and D-galactose, the reduction with sodium borohydride alters their structures. The specific stereochemistry of the resulting alcohol can influence whether the product retains optical activity, as seen with glucitol being optically active and the product from D-galactose being optically inactive.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: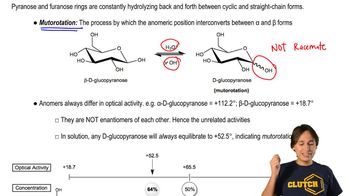


 7:19m
7:19m