NAME IT A prokaryotic cell hitched a ride to Earth on a space shuttle from some unknown planet. The organism is a psychrophile, an obligate halophile, and an obligate aerobe. Based on the characteristics of the microbe, describe the planet.
Ch. 6 - Microbial Growth
Chapter 6, Problem 2.4a
The optimum pH of Acidithiobacillus bacteria (pH 3) is _______________ times more acid than blood (pH 7).
a. 4
b. 10
c. 100
d. 1000
e. 10,000
 Verified step by step guidance
Verified step by step guidance1
Understand that pH is a logarithmic scale, which means each whole number change on the scale represents a tenfold change in acidity.
Calculate the difference in pH between the two environments: pH of blood (7) and pH of Acidithiobacillus bacteria (3).
Subtract the pH of Acidithiobacillus (3) from the pH of blood (7) to find the difference: 7 - 3.
Recognize that the difference in pH represents the power of 10 that indicates how many times more acidic one solution is compared to the other.
Use the result from the subtraction to determine the factor of acidity difference: 10 raised to the power of the difference.

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
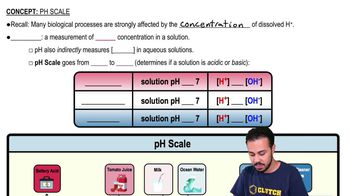
pH Scale
The pH scale measures the acidity or alkalinity of a solution, ranging from 0 to 14. A pH of 7 is neutral, values below 7 indicate acidity, and values above 7 indicate alkalinity. Each whole number change on the pH scale represents a tenfold change in hydrogen ion concentration, meaning that a solution with a pH of 3 is significantly more acidic than one with a pH of 7.
Recommended video:
Guided course
pH Scale
Hydrogen Ion Concentration
Hydrogen ion concentration is a key factor in determining the pH of a solution. As the pH decreases, the concentration of hydrogen ions increases exponentially. For example, a pH of 3 has 10,000 times more hydrogen ions than a pH of 7, which is crucial for understanding the relative acidity of different solutions.
Recommended video:
Guided course
Hydrogen Bonding
Acidithiobacillus Bacteria
Acidithiobacillus is a genus of bacteria known for thriving in extremely acidic environments, such as those with a pH of around 3. These bacteria play a significant role in biogeochemical cycles, particularly in the oxidation of iron and sulfur. Understanding their optimal pH helps in studying their ecological roles and potential applications in bioleaching and bioremediation.
Recommended video:
Guided course
Introduction to Bacteria
Related Practice
Textbook Question
112
views
Textbook Question
Use the following information to answer questions 1 and 2. Two culture media were inoculated with four different bacteria. After incubation, the following results were obtained:
<IMAGE>
Medium 1 is
a. selective.
b. differential.
c. both selective and differential.
127
views
Textbook Question
Use the following information to answer questions 1 and 2. Two culture media were inoculated with four different bacteria. After incubation, the following results were obtained:
<IMAGE>
Medium 2 is
a. selective.
b. differential.
c. both selective and differential.
118
views
