Here are the essential concepts you must grasp in order to answer the question correctly.
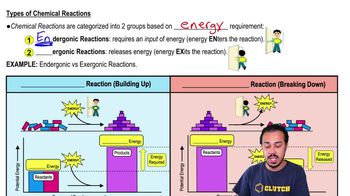
Exergonic Reactions
Exergonic reactions are chemical processes that release energy, typically in the form of heat or light. These reactions occur spontaneously and are characterized by a negative change in Gibbs free energy. An example of an exergonic reaction is cellular respiration, where glucose is broken down to release energy for cellular activities.
Recommended video:
Types of Chemical Reactions
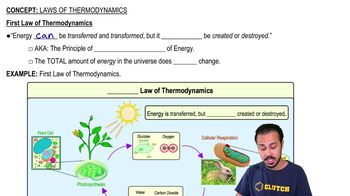
Thermodynamics
Thermodynamics is the branch of physics that deals with heat, work, and energy transformations. In microbiology, understanding thermodynamics is crucial for analyzing metabolic pathways and energy exchanges in living organisms. The laws of thermodynamics help explain how energy is conserved and transformed during biochemical reactions.
Recommended video:
Metabolism
Metabolism encompasses all chemical reactions that occur within a living organism to maintain life. It includes catabolic reactions, which break down molecules to release energy, and anabolic reactions, which use energy to build complex molecules. Understanding metabolism is essential for grasping how organisms utilize energy from their environment.
Recommended video:
Introduction to Metabolism
 Verified step by step guidance
Verified step by step guidance