Here are the essential concepts you must grasp in order to answer the question correctly.
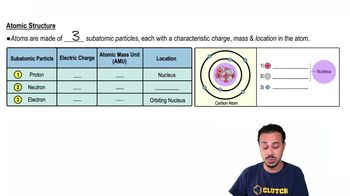
Atomic Structure
The atomic structure consists of protons, neutrons, and electrons. Protons carry a positive charge, electrons carry a negative charge, and neutrons are neutral, meaning they have no charge. Understanding this structure is essential for identifying the charge of different particles.
Recommended video:
Charge of Subatomic Particles
Subatomic particles are categorized by their electrical charge: protons are positively charged, electrons are negatively charged, and neutrons are uncharged. This distinction is crucial for understanding how these particles interact and form atoms, which are the building blocks of matter.
Recommended video:
Ions
Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a net electrical charge. Cations are positively charged ions (loss of electrons), while anions are negatively charged ions (gain of electrons). Recognizing the nature of ions helps in understanding their behavior in chemical reactions.
Recommended video:
 Verified step by step guidance
Verified step by step guidance