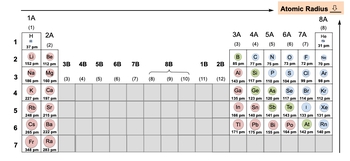
So atomic radius is the distance between an atom's nucleus and its outer electron shell, otherwise known as its valence shell. So here, if we take a look, we have our nucleus in the middle, and here we have our outer shell. The distance between the nucleus and the outer shell is our atomic radius r. Now remember, within the nucleus we have our protons and our neutrons; our protons are positively charged particles, neutrons are neutral, and the nucleus itself contains our protons and our neutrons.
Now, we're going to say here, going down a group, that the number of electrons increases because our shells get larger and larger, and they can hold more and more electrons. And we're going to say that the number of electron shells also increases. But, we're going to say moving across a period though, we're going to say that the number of electrons within the same shell also will increase. We're going to say increasing the number of shell electrons in the same shell causes a greater attraction with the nucleus. And what this does is it causes a slight decrease in our atomic radius.
So, we have these two forces at work; we're adding more electrons, and as a result our atom gets larger and larger with more and more shells. But as we add more and more shells, there’s going to be more electrons found within each of those shells. This is going to cause some issues with our atomic radius. So, there's an increase and a decrease, a type of phenomenon happening here with the increase of a number of electrons. The overall periodic trend is that as we move from left to right, so remember we're always heading towards the top right corner of the periodic table, our atomic radius will decrease.
So, click on to the next video and let's take a look at what this periodic table would look like in terms of atomic radius.