Here are the essential concepts you must grasp in order to answer the question correctly.
Concentration (m/v)%
Concentration expressed as mass/volume percentage (m/v)% indicates the mass of solute in grams per 100 mL of solution. For example, a 37 (m/v)% NaCl solution contains 37 grams of NaCl in 100 mL of solution. This measurement is crucial for understanding how much solute is present in a given volume of solution.
Recommended video:
Percent Concentrations Concept 1
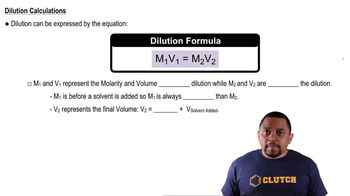
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. The dilution equation, C1V1 = C2V2, relates the initial concentration and volume (C1 and V1) to the final concentration and volume (C2 and V2). This concept is essential for calculating the new concentration after mixing solutions.
Recommended video:
Saturated Solution
A saturated solution is one in which the maximum amount of solute has been dissolved at a given temperature, resulting in an equilibrium between dissolved and undissolved solute. In this context, the initial saturated solution of NaCl at 37 (m/v)% provides a starting point for calculating the concentration after dilution, as it indicates the solute's maximum solubility.
Recommended video:
 Verified step by step guidance
Verified step by step guidance