Here are the essential concepts you must grasp in order to answer the question correctly.
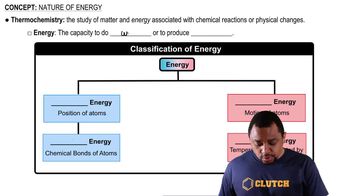
Energy Changes
Nuclear reactions involve changes in the nucleus of an atom, resulting in significant energy release, often millions of times greater than that of chemical reactions. In contrast, chemical reactions involve the rearrangement of electrons and typically release or absorb much smaller amounts of energy, measured in kilojoules.
Recommended video:
Reactants and Products
In nuclear reactions, the reactants and products can include different elements due to the transformation of one element into another through processes like fission or fusion. Chemical reactions, however, involve the same elements rearranging to form new compounds without changing the elemental identities.
Recommended video:
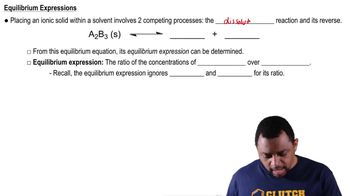
Solubility Product Constant (Ksp) Concept 2
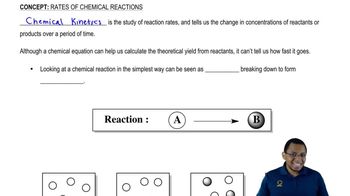
Rate of Reaction
Nuclear reactions occur at rates that are largely independent of temperature and pressure, often taking place over a wide range of conditions. In contrast, chemical reactions are significantly influenced by these factors, with reaction rates typically increasing with higher temperatures and concentrations.
Recommended video:
Rate of Reaction Concept 1