Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclide
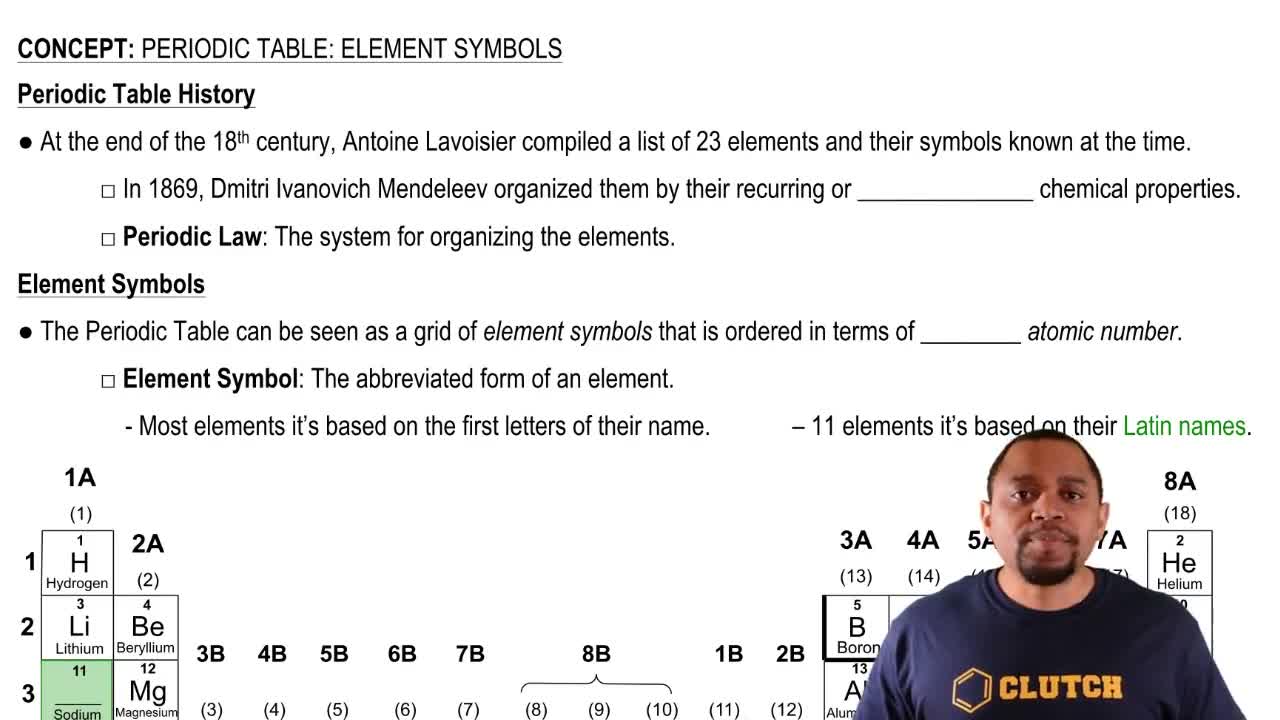
A nuclide is a distinct nuclear species characterized by its number of protons and neutrons. Each nuclide is represented by a symbol that includes its chemical element symbol, atomic number (number of protons), and mass number (total number of protons and neutrons). Understanding nuclides is essential for identifying elements in decay series.
Decay Series
A decay series is a sequence of nuclear reactions in which an unstable nuclide transforms into a series of other nuclides until a stable nuclide is formed. Each step in the series involves the emission of radiation, such as alpha or beta particles, and results in the formation of new nuclides. Recognizing the order and types of decay is crucial for determining the symbols of the nuclides involved.
Recommended video:
Symbol Representation
The symbol representation of a nuclide includes the element's chemical symbol, the atomic number as a subscript, and the mass number as a superscript. For example, the symbol for carbon-14 is written as ¹⁴C, where 14 is the mass number and C is the chemical symbol for carbon. Mastery of this notation is vital for accurately identifying and writing the symbols of nuclides in a decay series.
Recommended video:
Periodic Table: Symbols Concept
 Verified step by step guidance
Verified step by step guidance