Here are the essential concepts you must grasp in order to answer the question correctly.
Acidity and Acid Strength
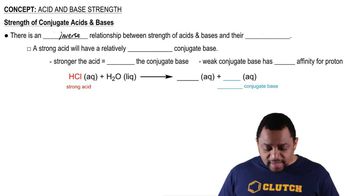
Acidity refers to the ability of a substance to donate protons (H⁺ ions) in a solution. The strength of an acid is determined by its dissociation constant (Ka), which measures how completely an acid ionizes in water. Stronger acids have higher Ka values and more readily release protons, making them more effective in chemical reactions.
Recommended video:
Acid and Base Strength Concept 3
Electrostatic Potential Maps
Electrostatic potential maps visually represent the distribution of electron density around a molecule. Areas of high electron density are shown in blue, while low density is indicated in red. These maps help identify regions of partial positive and negative charges, which are crucial for understanding reactivity and acidity, particularly in determining the most acidic hydrogen in a molecule.
Recommended video:
The Atom (Simplified) Example 2
Identifying Acidic Hydrogens
In organic acids, the most acidic hydrogen is typically attached to a functional group that can stabilize the resulting anion after deprotonation. For acetic acid (CH₃COOH), the hydrogen on the carboxylic group (-COOH) is the most acidic, while in ethyl alcohol (CH₃CH₂OH), the hydroxyl group (-OH) contains the acidic hydrogen. The stability of the anion formed after losing this hydrogen influences the overall acidity.
Recommended video:
Hydrogenation Reactions Example 1

 Verified step by step guidance
Verified step by step guidance