Pentoses also exist in a ring form, but they most commonly occur as furanose rings. d-Ribose exists in its furanose ring form in the nucleic acid RNA. Using the structure of d-ribose from Table 6.1, draw the furanose form of ß-d-ribose.
Identify a disaccharide that fits each of the following descriptions:
(a) ordinary table sugar
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
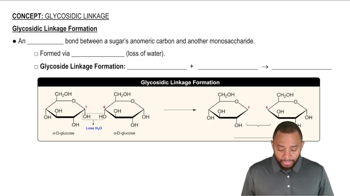
Disaccharides

Sucrose

Glycosidic Bond
For each of the following disaccharides, name the glycosidic bond and draw the monosaccharide units produced by hydrolysis: (a) <IMAGE>
ALLIED Health Lactulose is a disaccharide used in the treatment of chronic constipation. Its formal name is galactose ß (1→4) fructose.
(a) Draw the structure of lactulose.
ALLIED Health Based on the sweetness index in Table 6.2, if you tasted a drop of each of the syrups below, which would taste the sweetest?
(a) light corn syrup (100% glucose)
ALLIED Health If one sweetener packet of Splenda, Sweet’N Low, or Equal has the same sweetness as two tablespoons of sugar, according to Table 6.2, which of the packets contains the smallest amount of the sweetener?
Describe the similarities and differences of the following polysaccharides:
(a) amylose and amylopectin
