Here are the essential concepts you must grasp in order to answer the question correctly.
Periodic Table
The periodic table is a systematic arrangement of chemical elements, organized by increasing atomic number. Elements are grouped into rows called periods and columns known as groups, which reflect their similar properties. Understanding the layout of the periodic table is essential for identifying the characteristics of elements, including their group and period numbers.
Recommended video:
Periodic Table: Classifications
Group Number
The group number in the periodic table indicates the number of valence electrons in the outer shell of an element, which influences its chemical behavior. Elements in the same group often exhibit similar chemical properties. For example, aluminum is located in Group 13, which signifies it has three valence electrons.
Recommended video:
Calculate Oxidation Numbers
Period Number
The period number represents the horizontal rows in the periodic table and indicates the highest energy level of electrons in an atom of the element. As you move down the periods, the number of electron shells increases. Aluminum is found in Period 3, meaning it has three electron shells.
Recommended video:
Calculate Oxidation Numbers
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution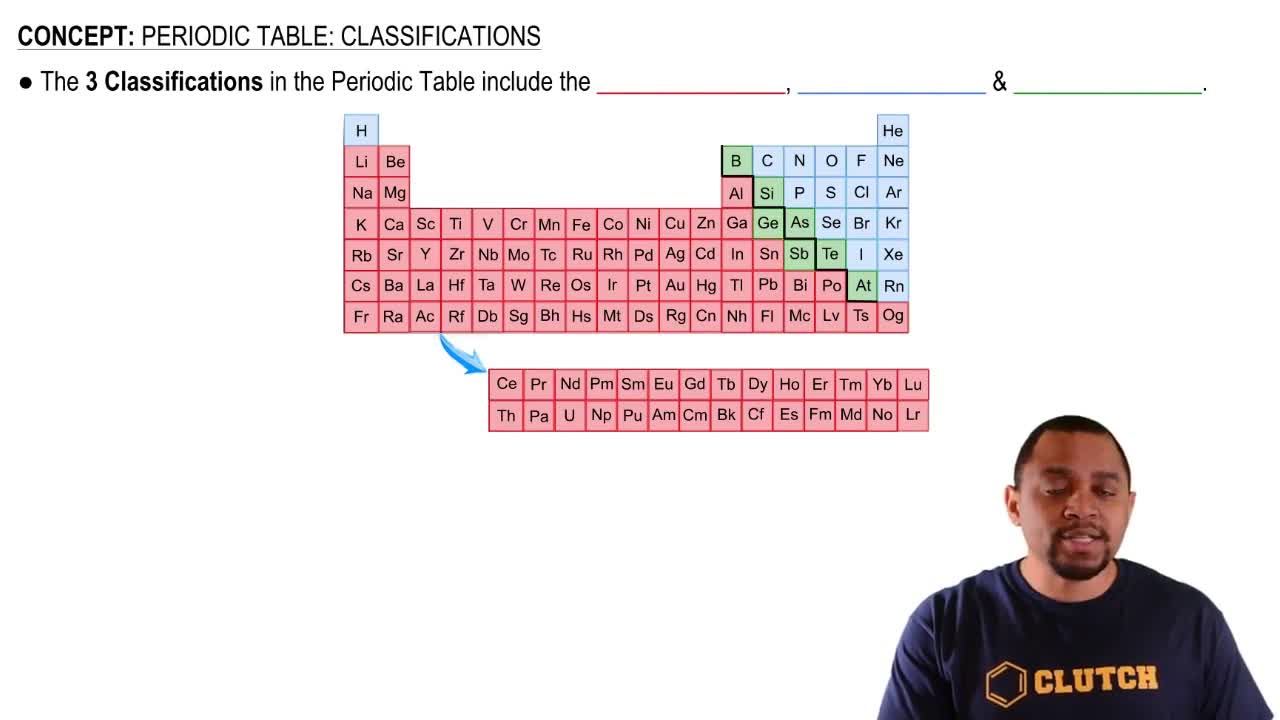



 5:33m
5:33m