Here are the essential concepts you must grasp in order to answer the question correctly.
Noble Gases
Noble gases are a group of chemical elements in Group 18 of the periodic table, characterized by their lack of reactivity due to having a full valence shell of electrons. This group includes helium, neon, argon, krypton, xenon, and radon. Helium, the lightest noble gas, is often used in balloons and as a cooling medium in cryogenics.
Recommended video:
Periodic Table Groups
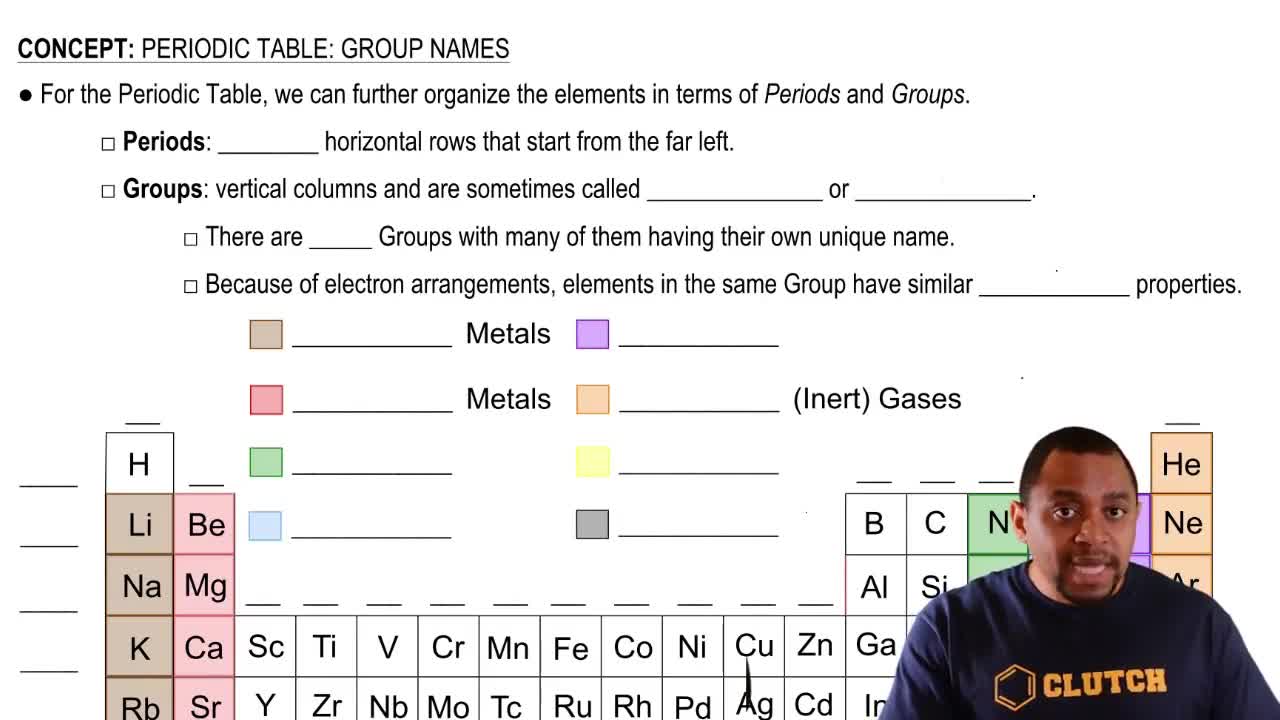
The periodic table is organized into columns called groups, which contain elements with similar chemical properties. Each group is numbered from 1 to 18, with Group 1 containing alkali metals and Group 18 containing noble gases. Understanding the group number helps predict the behavior and reactivity of elements.
Recommended video:
Periodic Table: Group Names
Element Positioning
The positioning of elements in the periodic table is determined by their atomic number, which is the number of protons in an atom's nucleus. Elements are arranged in increasing order of atomic number, and their placement in specific groups and periods reflects their electronic configuration and chemical properties. For example, helium is located in period 1 and group 18.
Recommended video:
Valence Electrons of Elements (Simplified) Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 5:33m
5:33m