Textbook Question
Which electron is, on average, farther from the nucleus: an electron in a 3p orbital or an electron in a 4p orbital?
852
views
 Verified step by step guidance
Verified step by step guidance

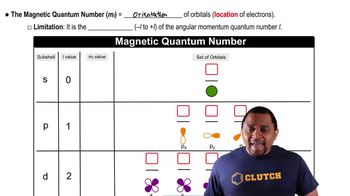

Which electron is, on average, farther from the nucleus: an electron in a 3p orbital or an electron in a 4p orbital?
What are the possible values of l for each given value of n? a. 1 b. 2 c. 3 d. 4
What are the possible values of ml for each value of l? a. 0
What are the possible values of ml for each value of l? c. 2 d. 3
Which set of quantum numbers cannot occur together to specify an orbital? a. n = 2, l = 1, ml = -1 b. n = 3, l = 2, ml = 0 c. n = 3, l = 3, ml = 2 d. n = 4, l = 3, ml = 0
Which combinations of n and l represent real orbitals, and which do not exist? a. 1s b. 2p c. 4s d. 2d