Here are the essential concepts you must grasp in order to answer the question correctly.
Alkane Structure
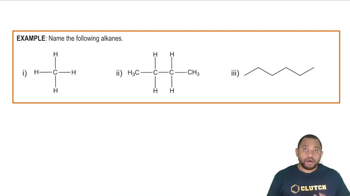
Alkanes are saturated hydrocarbons consisting solely of carbon and hydrogen atoms, connected by single bonds. Their general formula is CnH2n+2, where 'n' is the number of carbon atoms. Understanding the structure of alkanes is crucial for predicting their reactivity in substitution reactions, as it determines how many hydrogen atoms can be replaced.
Recommended video:
Substitution Reactions
Substitution reactions involve the replacement of one atom or group in a molecule with another atom or group. In the case of alkanes, this typically occurs when a halogen, such as bromine, reacts with the alkane, resulting in the substitution of a hydrogen atom. This process is often initiated by heat or light, which helps to break the bonds in the reactants.
Recommended video:
Alcohol Reactions: Substitution Reactions
Monosubstitution Products
In a monosubstitution reaction, only one hydrogen atom in the alkane is replaced by a halogen atom. For example, when ethane (CH3CH3) reacts with bromine (Br2), the possible products include bromoethane (C2H5Br) and hydrogen bromide (HBr). Identifying the products requires understanding the positions where substitution can occur, leading to different isomers.
Recommended video: