Predict a likely mode of decay for each unstable nuclide. c. In-132
A radioactive sample contains 2.35 g of an isotope with a halflife of 3.8 days. What mass of the isotope remains after 7.0 days?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
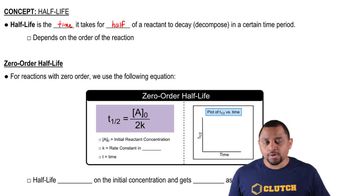
Key Concepts
Radioactive Decay

Half-Life
Exponential Decay Formula

Which nuclide in each pair would you expect to have the longer half-life? a. Cs-149 or Cs-139 b. Fe-45 or Fe-52
One of the nuclides in spent nuclear fuel is U-235, an alpha emitter with a half-life of 703 million years. How long will it take for the amount of U-235 to reach 10.0% of its initial amount?
A sample of F-18 has an initial decay rate of 1.5⨉105/s. How long will it take for the decay rate to fall to 2.5⨉103/s? (F-18 has a half-life of 1.83 hours.)
A mammoth skeleton has a carbon-14 decay rate of 0.48 disintegration per minute per gram of carbon (0.48 dis/min • g C). When did the mammoth live? (Assume that living organisms have a carbon-14 decay rate of 15.3 dis/min • g C and that carbon- 14 has a half-life of 5715 yr.)
A rock from Australia contains 0.438 g of Pb-206 for every 1.00 g of U-238. Assuming that the rock did not contain any Pb-206 at the time of its formation, how old is the rock?
