Here are the essential concepts you must grasp in order to answer the question correctly.
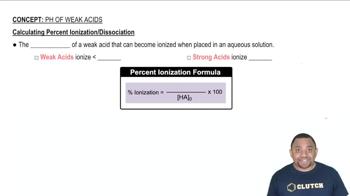
Ionization of Weak Acids
Weak acids, like HNO2 (nitrous acid), do not fully ionize in solution. The degree of ionization depends on the concentration of the acid and the presence of other ions in the solution. In pure water, HNO2 will ionize to a certain extent, but this ionization can be affected by the addition of other solutes.
Recommended video:
Calculating Percent Ionization of Weak Acids
Common Ion Effect
The common ion effect occurs when a salt containing an ion that is also produced by the weak acid is added to the solution. This increases the concentration of that ion, shifting the equilibrium of the weak acid's ionization reaction to the left, thereby reducing the degree of ionization. For example, adding NaNO2 introduces NO2- ions, which will suppress the ionization of HNO2.
Recommended video:
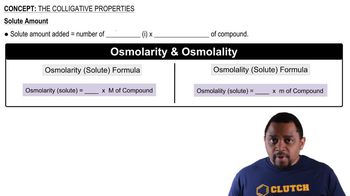
Colligative Properties
Colligative properties depend on the number of solute particles in a solution rather than their identity. When a non-volatile solute like NaCl or KNO3 is added to water, it can affect the physical properties of the solution, such as vapor pressure and boiling point. However, in the context of weak acid ionization, the presence of ions from strong electrolytes can influence the ionization behavior of weak acids.
Recommended video: