Textbook Question
Based on molecular structure, arrange the oxyacids in order of increasing acid strength. Explain your choice. HClO3, HIO3, HBrO3
762
views



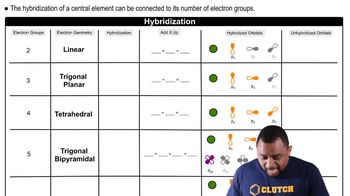
Based on molecular structure, arrange the oxyacids in order of increasing acid strength. Explain your choice. HClO3, HIO3, HBrO3
Which is a stronger base, S2– or Se2–? Explain.
Classify each species as either a Lewis acid or a Lewis base. a. Fe3+ b. BH3 c. NH3 d. F-
Classify each species as either a Lewis acid or a Lewis base. b. OH–
Classify each species as either a Lewis acid or a Lewis base. c. B(OH)3
Classify each species as either a Lewis acid or a Lewis base. d. CN–