In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning. b. CH3CH2CH2OH or CH3OH
Which compound would you expect to have greater surface tension: acetone [(CH3)2CO] or water (H2O)? Explain.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Surface Tension
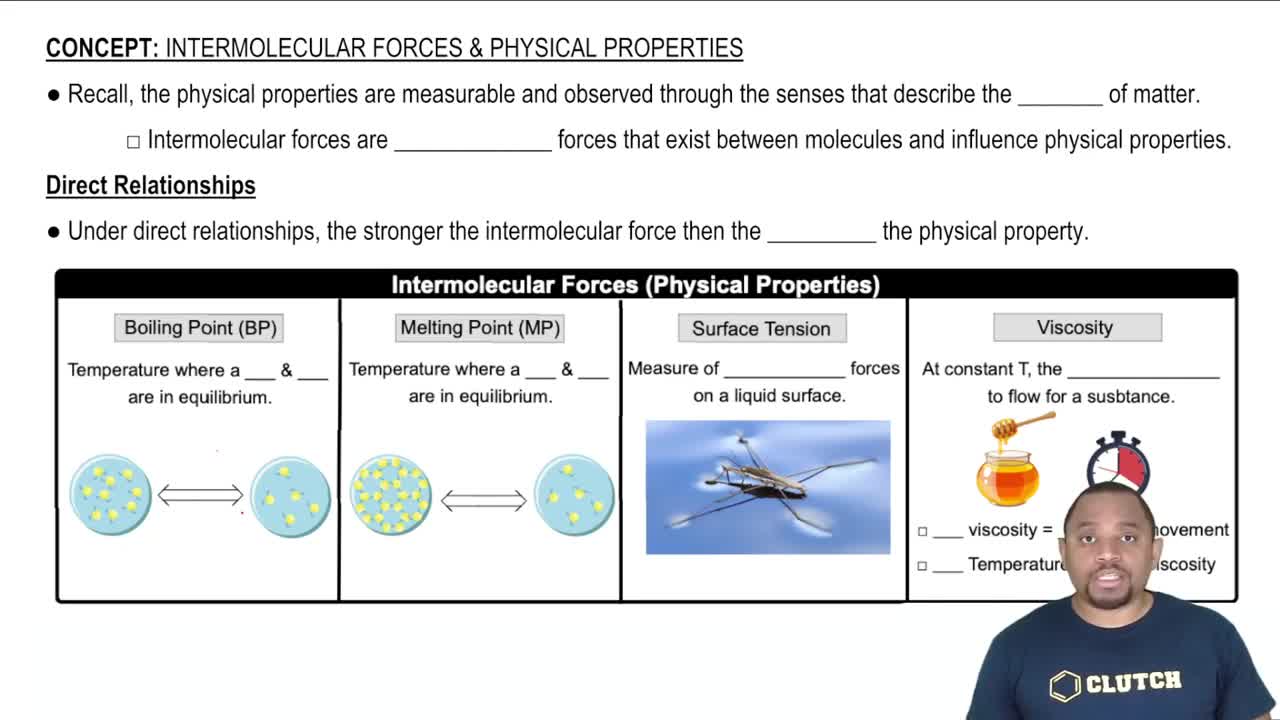
Hydrogen Bonding

Molecular Structure and Polarity

Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. a. CCl4 and H2O b. KCl and H2O c. Br2 and CCl4
Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. d. CH3CH2OH and H2O
Explain why the viscosity of multigrade motor oils is less temperature-dependent than that of single-grade motor oils.
When a thin glass tube is put into water, the water rises 1.4 cm. When the same tube is put into hexane, the hexane rises only 0.4 cm. Explain.
Which evaporates more quickly: 100 mL of water (H2O) in a beaker or 100 mL of acetone [(CH3)2CO] in an identical beaker under identical conditions? Is the vapor pressure of the two substances different? Explain.
