Textbook Question
A molecule with the formula AB3 has a trigonal pyramidal geometry. How many electron groups are on the central atom (A)?
3053
views
 Tro 6th Edition
Tro 6th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 34c
Problem 34c



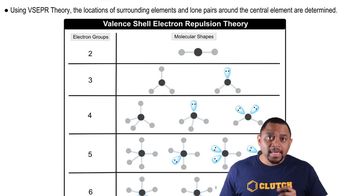
A molecule with the formula AB3 has a trigonal pyramidal geometry. How many electron groups are on the central atom (A)?
A molecule with the formula AB2 has a linear geometry. How many electron groups are on the central atom?
Determine the electron geometry, molecular geometry, and idealized bond angles for each molecule. In which cases do you expect deviations from the idealized bond angle?
a. CI4
b. NCl3
c. OF2
d. H2S
Determine the electron geometry, molecular geometry, and idealized bond angles for each molecule. In which cases do you expect deviations from the idealized bond angle?
a. CS2
b. SCl2
c. CHF3
d. PF3
Which species has the smaller bond angle, H3O+ or H2O? Explain.