Here are the essential concepts you must grasp in order to answer the question correctly.
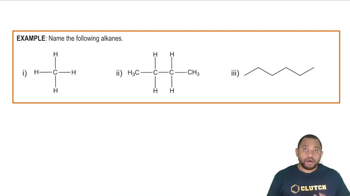
Alkane Structure
Alkanes are saturated hydrocarbons consisting only of carbon and hydrogen atoms, connected by single bonds. Their general formula is CnH2n+2, where 'n' is the number of carbon atoms. Understanding the structure of alkanes is crucial for predicting the products of substitution reactions, as the position of the hydrogen atom being replaced will influence the resulting compound.
Recommended video:
Substitution Reactions
Substitution reactions involve the replacement of one atom or group in a molecule with another atom or group. In the context of alkanes, monosubstitution refers to the replacement of a single hydrogen atom with a halogen (like Cl or Br) during a reaction with halogens. This process is fundamental in organic chemistry, as it leads to the formation of haloalkanes, which have different properties than the original alkane.
Recommended video:
Alcohol Reactions: Substitution Reactions
Regioselectivity
Regioselectivity refers to the preference of a chemical reaction to occur at one location over another in a molecule. In alkane substitution reactions, the position where the hydrogen is replaced can lead to different products, especially in branched alkanes. Understanding regioselectivity helps predict the major products formed during the reaction, as certain positions may be more favorable for substitution due to steric and electronic factors.
 Verified step by step guidance
Verified step by step guidance