Here are the essential concepts you must grasp in order to answer the question correctly.
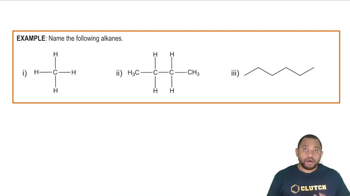
Alkanes
Alkanes are saturated hydrocarbons consisting only of carbon (C) and hydrogen (H) atoms, connected by single bonds. They follow the general formula CnH2n+2, where 'n' is the number of carbon atoms. Alkanes are characterized by their relatively low reactivity and are commonly found in natural gas and petroleum.
Recommended video:
Structural Isomers
Structural isomers are compounds that have the same molecular formula but differ in the arrangement of atoms. In the case of alkanes, this means that different structural forms can exist for the same number of carbon atoms, leading to variations in physical and chemical properties. Understanding structural isomers is crucial for drawing accurate chemical structures.
Recommended video:
IUPAC Nomenclature
IUPAC nomenclature is a systematic method for naming chemical compounds, ensuring that each compound has a unique and descriptive name. For alkanes, the name reflects the longest continuous carbon chain and any substituents. In the case of 3-ethylhexane, the name indicates a hexane chain with an ethyl group attached to the third carbon, guiding the drawing of its structure.
Recommended video:
 Verified step by step guidance
Verified step by step guidance