Here are the essential concepts you must grasp in order to answer the question correctly.
Hydrogenation
Hydrogenation is a chemical reaction that involves the addition of hydrogen (H2) to an unsaturated compound, typically an alkene or alkyne, resulting in the formation of a saturated compound. This process is commonly used in organic chemistry to convert liquid vegetable oils into solid fats, such as margarine, by breaking double or triple bonds.
Recommended video:
Catalysts in Hydrogenation
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. In hydrogenation reactions, catalysts such as palladium, platinum, or nickel are often used to facilitate the addition of hydrogen to the unsaturated bonds, making the reaction more efficient and allowing it to occur under milder conditions.
Recommended video:
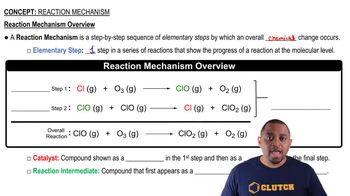
Reaction Mechanism
The reaction mechanism describes the step-by-step sequence of elementary reactions by which overall chemical change occurs. In hydrogenation, the mechanism typically involves the adsorption of hydrogen and the unsaturated compound onto the catalyst's surface, followed by the formation of new bonds and desorption of the saturated product, which is crucial for understanding how the reaction proceeds.
Recommended video:
Reaction Mechanism Overview
 Verified step by step guidance
Verified step by step guidance