A typical nuclear reactor produces about 1.0 MW of power per day. What is the minimum rate of mass loss required to produce this much energy?
Ch.21 - Radioactivity & Nuclear Chemistry
Chapter 21, Problem 90
The nuclide 6Li reacts with 2H to form two identical particles. Identify the particles.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Reactions
Nuclear reactions involve changes in the nucleus of an atom, resulting in the transformation of one element into another or the release of energy. In this case, the reaction between lithium-6 and deuterium (a hydrogen isotope) leads to the formation of new particles, illustrating how nuclear interactions can produce different nuclides.
Recommended video:
Guided course

Nuclear Binding Energy
Isotopes
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. In the question, lithium-6 (with 3 protons and 3 neutrons) and deuterium (with 1 proton and 1 neutron) are isotopes that participate in the reaction, highlighting the importance of understanding isotopic composition in nuclear chemistry.
Recommended video:
Guided course

Isotopes
Conservation of Mass and Charge
In nuclear reactions, both mass and charge must be conserved. This principle dictates that the total mass and charge of the reactants must equal the total mass and charge of the products. Identifying the two identical particles formed from the reaction requires applying this conservation law to ensure that the resulting particles maintain the balance of mass and charge from the original nuclides.
Recommended video:
Guided course
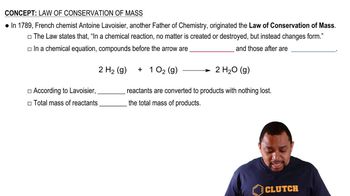
Law of Conservation of Mass
Related Practice
Textbook Question
500
views
Textbook Question
Find the binding energy in an atom of 3He, which has a mass of 3.016030 amu.
877
views
Textbook Question
The nuclide 247Es can be made by bombardment of 238U in a reaction that emits five neutrons. Identify the bombarding particle.
601
views
1
rank
Textbook Question
The half-life of 238U is 4.5⨉109 yr. A sample of rock of mass 1.6 g produces 29 dis/s. Assuming all the radioactivity is due to 238U, find the percent by mass of 238U in the rock.
1213
views
Textbook Question
The half-life of 232Th is 1.4⨉1010 yr. Find the number of disintegrations per hour emitted by 1.0 mol of 232Th.
762
views
1
comments
Textbook Question
The nuclide 18F decays by both electron capture and β+ decay. Find the difference in the energy released by these two processes. The atomic masses are 18F = 18.000950 and 18O = 17.9991598.
467
views
