Here are the essential concepts you must grasp in order to answer the question correctly.
Strong vs. Weak Acids
Strong acids, like HCl, completely dissociate in solution, resulting in a higher concentration of hydrogen ions (H+) and a lower initial pH. In contrast, weak acids, such as HF, only partially dissociate, leading to a higher initial pH due to a lower concentration of H+ ions in solution.
Recommended video:
Weak Acid-Strong Base Titration Curve
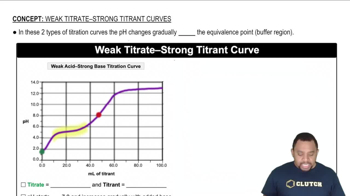
Titration Curves
A titration curve plots pH against the volume of titrant added. The shape of the curve is influenced by the strength of the acid and base involved. For strong acid-strong base titrations, the initial pH is low, while weak acid-strong base titrations show a more gradual increase in pH, reflecting the acid's partial dissociation.
Recommended video:
Acid-Base Titration Curves
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 (very acidic) to 14 (very basic), with 7 being neutral. The pH is logarithmically related to the concentration of H+ ions; thus, a small change in pH corresponds to a significant change in H+ concentration, which is crucial for understanding the initial conditions of the titration.
Recommended video: