Calculate the percent ionization of a formic acid solution having the given concentration. c. 0.100 M
Find the pH of each mixture of acids. a. 0.115 M in HBr and 0.125 M in HCHO2
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Strong vs. Weak Acids
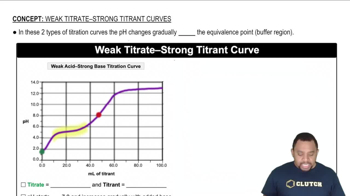
pH Calculation

Acid-Base Mixtures

Calculate the percent ionization of a formic acid solution having the given concentration. d. 0.0500 M
A 0.148 M solution of a monoprotic acid has a percent ionization of 1.55%. Determine the acid ionization constant (Ka) for the acid.
Find the pH of each mixture of acids. b. 0.150 M in HNO2 and 0.085 M in HNO3 c. 0.185 M in HCHO2 and 0.225 M in HC2H3O2 d. 0.050 M in acetic acid and 0.050 M in hydrocyanic acid
Find the pH of each mixture of acids. a. 0.075 M in HNO3 and 0.175 M in HC7H5O2 b. 0.020 M in HBr and 0.015 M in HClO4 c. 0.095 M in HF and 0.225 M in HC6H5O d. 0.100 M in formic acid and 0.050 M in hypochlorous acid
For each strong base solution, determine [OH–], [H3O+], pH, and pOH. a. 0.15 M NaOH b. 1.5×10–3 M Ca(OH)2 c. 4.8×10–4 M Sr(OH)2 d. 8.7×10–5 M KOH
