Textbook Question
Barium has a density of 3.59 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a barium atom.
2592
views



Barium has a density of 3.59 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a barium atom.
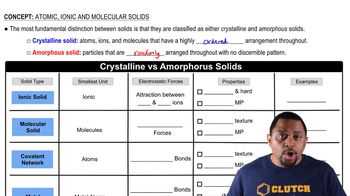
Identify each solid as molecular, ionic, or atomic. a. CaCl2(s)
Identify each solid as molecular, ionic, or atomic. b. CO2(s)
Which solid has the highest melting point? Why? C(s, diamond), Kr(s), NaCl(s), H2O(s)
Which solid in each pair has the higher melting point and why?
a. TiO2(s) or HOOH(s)
b. CCl4(s) or SiCl4(s)
c. Kr(s) or Xe(s)
Which solid in each pair has the higher melting point and why? d. NaCl(s) or CaO(s)