Textbook Question
Rhodium has a density of 12.41 g/cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.
2271
views
 Verified step by step guidance
Verified step by step guidance


Rhodium has a density of 12.41 g/cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.
Barium has a density of 3.59 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a barium atom.
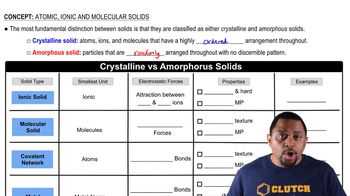
Identify each solid as molecular, ionic, or atomic. a. CaCl2(s)
Identify each solid as molecular, ionic, or atomic. d. I2(s)
Which solid has the highest melting point? Why? C(s, diamond), Kr(s), NaCl(s), H2O(s)
Which solid in each pair has the higher melting point and why?
a. TiO2(s) or HOOH(s)
b. CCl4(s) or SiCl4(s)
c. Kr(s) or Xe(s)