Here are the essential concepts you must grasp in order to answer the question correctly.
Orbital Diagrams
Orbital diagrams visually represent the distribution of electrons in an atom's orbitals. Each orbital can hold a maximum of two electrons with opposite spins, and the diagram helps illustrate how electrons fill these orbitals according to the Aufbau principle, Hund's rule, and the Pauli exclusion principle. Understanding how to construct these diagrams is essential for determining the electron configuration of ions.
Recommended video:
Electron Orbital Diagrams
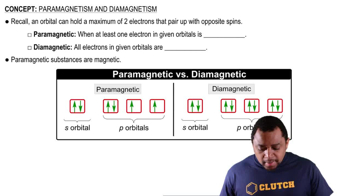
Diamagnetism and Paramagnetism
Diamagnetism and paramagnetism are properties that describe how materials respond to magnetic fields. Diamagnetic substances have all their electrons paired, resulting in no net magnetic moment and a weak repulsion from magnetic fields. In contrast, paramagnetic substances have unpaired electrons, leading to a net magnetic moment and a weak attraction to magnetic fields, which is crucial for identifying the magnetic properties of ions.
Recommended video:
Paramagnetism and Diamagnetism
Electron Configuration of Ions
The electron configuration of ions is derived from the neutral atom's configuration, adjusted for the loss or gain of electrons. For cations, electrons are removed from the outermost orbitals first, which can affect the overall electron arrangement. Understanding how to write the electron configuration for ions is vital for constructing their orbital diagrams and determining their magnetic properties.
Recommended video:
Anion Electron Configuration
 Verified step by step guidance
Verified step by step guidance