Textbook Question
Assign oxidation states to each atom in each element, ion, or compound. Cl2, Fe3+, CuCl2, CH4, Cr2O72–, HSO4–
954
views
 Verified step by step guidance
Verified step by step guidance


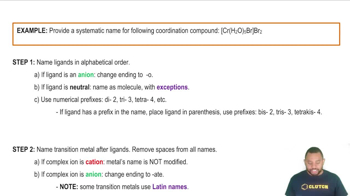
Assign oxidation states to each atom in each element, ion, or compound. Cl2, Fe3+, CuCl2, CH4, Cr2O72–, HSO4–
What is the oxidation state of Cr in each compound? a. CrO
What is the oxidation state of Cr in each compound? b. CrO3
What is the oxidation state of Cl in each ion? a. ClO- b. ClO2- c. ClO3- d. ClO4-
Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and the reducing agent. a. 4 Li(s) + O2(g) → 2 Li2O(s) b. Mg(s) + Fe2+(aq) → Mg2+(aq) + Fe(s)
Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and the reducing agent. c. Pb(NO3)2(aq) + Na2SO4(aq) → PbSO4(s) + 2 NaNO3(aq) d. HBr(aq) + KOH(aq) → H2O(l) + KBr(aq)