Here are the essential concepts you must grasp in order to answer the question correctly.
Molarity
Molarity (M) is a measure of concentration defined as the number of moles of solute per liter of solution. It is expressed in moles per liter (mol/L) and is crucial for understanding how much solute is present in a given volume of solution. In this problem, the initial molarity of the glucose solution is given, which will be used to calculate the final concentration after dilution.
Recommended video:
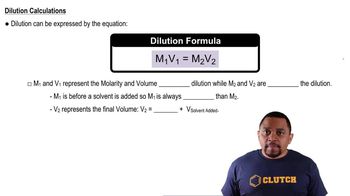
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. The relationship between the initial and final concentrations and volumes is described by the dilution equation: C1V1 = C2V2, where C1 and V1 are the initial concentration and volume, and C2 and V2 are the final concentration and volume. This concept is essential for solving the problem as it allows us to find the new molarity after dilution.
Recommended video:
Volume Conversion
Volume conversion is the process of changing the volume measurement from one unit to another, often necessary in chemistry calculations. In this case, the initial volume of the glucose solution is given in milliliters (mL), while the final volume after dilution is also in mL. Understanding how to work with these units is important for accurately applying the dilution formula and ensuring that the final molarity is calculated correctly.
Recommended video:
Common Conversion Factors