Textbook Question
The elemental mass percent composition of ibuprofen (a nonsteroidal anti-inflammatory drug [NSAID]) is 75.69% C, 8.80% H, and 15.51% O. Determine the empirical formula of ibuprofen.
1497
views
 Verified step by step guidance
Verified step by step guidance


The elemental mass percent composition of ibuprofen (a nonsteroidal anti-inflammatory drug [NSAID]) is 75.69% C, 8.80% H, and 15.51% O. Determine the empirical formula of ibuprofen.
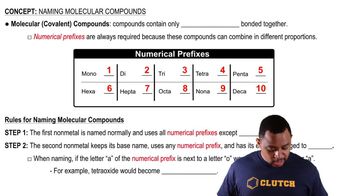
Write the formula for each molecular compound. a. boron tribromide b. dichlorine monoxide c. xenon tetrafluoride d. carbon tetrabromide
Determine the number of each type of atom in each formula. a. Mg3(PO4)2 b. BaCl2 c. Fe(NO2)2 d. Ca(OH)2
Determine the number of each type of atom in each formula. a. Ca(NO2)2 d. Mg(HCO3)2
Determine the number of each type of atom in each formula. b. CuSO4 d. Mg(HCO3)2