Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed when atoms transfer electrons, resulting in the formation of charged ions. Typically, these compounds consist of a metal and a non-metal, where the metal loses electrons to become a positively charged cation, and the non-metal gains electrons to become a negatively charged anion. The electrostatic attraction between these oppositely charged ions holds the compound together.
Recommended video:
Molecular Compounds
Molecular compounds are formed when two or more non-metal atoms share electrons through covalent bonds. These compounds consist of discrete molecules, where the atoms are held together by shared pairs of electrons. Molecular compounds often have lower melting and boiling points compared to ionic compounds and can exist in various states (solid, liquid, gas) at room temperature.
Recommended video:
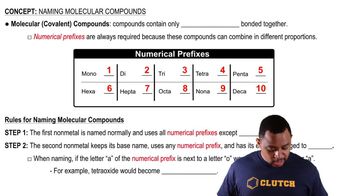
Naming Molecular Compounds
Electronegativity and Bonding
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a bond. In classifying compounds, understanding the difference in electronegativity between the atoms involved helps determine whether a bond is ionic or covalent. A large difference in electronegativity typically indicates an ionic bond, while a small difference suggests a covalent bond, which is crucial for identifying whether a compound is ionic or molecular.
Recommended video: