Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed when atoms transfer electrons, resulting in the formation of charged ions. Typically, these compounds consist of a metal and a non-metal, where the metal donates electrons and the non-metal accepts them. The electrostatic attraction between the oppositely charged ions creates a strong bond, leading to high melting and boiling points.
Recommended video:
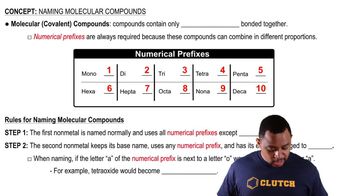
Molecular Compounds
Molecular compounds are formed when two or more non-metals share electrons through covalent bonds. These compounds usually have lower melting and boiling points compared to ionic compounds and can exist in various states (solid, liquid, gas) at room temperature. The properties of molecular compounds are influenced by the types of atoms involved and the shape of the molecules.
Recommended video:
Naming Molecular Compounds
Electronegativity and Bonding
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a bond. In classifying compounds, understanding the electronegativity of the constituent elements helps determine whether a bond is ionic or covalent. A significant difference in electronegativity between two atoms typically indicates an ionic bond, while similar values suggest a covalent bond, which is crucial for identifying molecular compounds.
Recommended video: