Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Structure
Molecular structure refers to the arrangement of atoms within a molecule, including the connectivity and spatial orientation of these atoms. Understanding molecular structure is crucial for determining whether two molecules are identical or different, as even slight variations in arrangement can lead to distinct chemical properties.
Recommended video:
Stereoisomerism
Stereoisomerism is a form of isomerism where molecules have the same molecular formula and connectivity but differ in the spatial arrangement of atoms. Enantiomers are a specific type of stereoisomers that are non-superimposable mirror images of each other, which is essential for understanding the differences between molecules in the given pairs.
Recommended video:
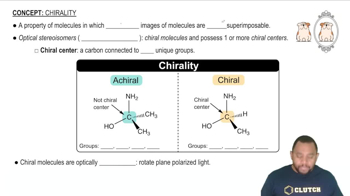
Chirality
Chirality is a property of a molecule that makes it non-superimposable on its mirror image, much like left and right hands. A chiral molecule typically has at least one carbon atom bonded to four different substituents, leading to the formation of two enantiomers. Recognizing chirality is vital for determining whether the molecules in question are the same or enantiomers.
Recommended video:
 Verified step by step guidance
Verified step by step guidance