What is the mass, in grams, of each elemental sample? a. 2.3×10–3 mol Sb b. 0.0355 mol Ba d. 1.3 mol W
An automobile gasoline tank holds 21 kg of gasoline. When the gasoline burns, 84 kg of oxygen is consumed, and carbon dioxide and water are produced. What is the total combined mass of carbon dioxide and water that is produced?

Verified Solution
Key Concepts
Law of Conservation of Mass
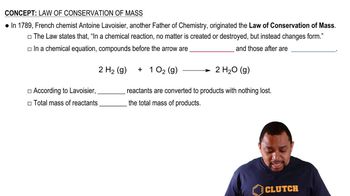
Stoichiometry

Combustion Reaction

Identify the elements that have molecules as their basic units. a. hydrogen b. iodine c. lead d. oxygen
A hydrogen-filled balloon is ignited and 1.50 g of hydrogen is reacted with 12.0 g of oxygen. How many grams of water vapor form? (Assume that water vapor is the only product.)
Two samples of carbon tetrachloride are decomposed into their constituent elements. One sample produces 38.9 g of carbon and 448 g of chlorine, and the other sample produces 14.8 g of carbon and 134 g of chlorine. Are these results consistent with the law of definite proportions? Explain your answer.
The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produces 28.8 g of sodium upon decomposition. How much fluorine (in grams) forms?
