Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn . b. C(s) + H2O(g) → CO(g) + H2(g)
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn . a. 3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Entropy (ΔS)

Standard Entropy Values

Rationalizing ΔS°rxn Sign
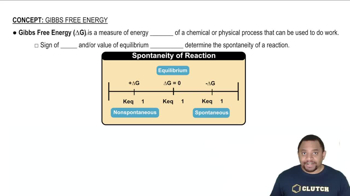
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn. c. CO(g) + H2O(g) → H2(g) + CO2(g)
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn. d. 2 H2S(g) + 3 O2(g) → 2 H2O(l) + 2 SO2(g)
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn. b. Cr2O3(s) + 3 CO(g) → 2 Cr(s) + 3 CO2(g)
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn. c. SO2(g) + 1/2 O2(g) → SO3(g)
Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn. d. N2O4(g) + 4 H2(g) → N2(g) + 4 H2O(g)
