Open Question
Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base in aqueous solution according to the Arrhenius definition. a. NaOH(aq) b. H2SO4(aq) c. HBr(aq) d. Sr(OH)2(aq)



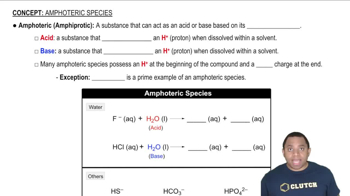
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. b. NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. d. C5H5N(aq) + H2O(l) ⇌ C5H5NH+(aq) + OH–(aq)
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. c. CO32–(aq) + H2O(l) ⇌ HCO3–(aq) + OH–(aq)
Write the formula for the conjugate base of each acid. a. HCl
Write the formula for the conjugate base of each acid. b. H2SO3